Application Note: SEC–MALS Analysis of Exosomes Using the Shodex OHpak SB-806 HQ
Once thought of as garbage bins, extracellular membrane vesicles full of cellular remnants responsible for the possible spread of many diseases, exosomes are viewed now as potential vehicles for regenerative medicine and targeted therapies for chronic and degenerative diseases, certain genetic disorders, musculoskeletal pain, and even Alzheimer’s disease. Targeted therapies may use a variety of targeting or signaling molecules such as RNA (messenger RNA and small interfering RNA, for example), DNA fragments, peptides, proteins, and lipids (1).
However, it is important to separate and purify the desired exosome from impurities during production. In this example of exosome (EV) analysis, the EV preparation process from cell culture supernatant was followed by a combination of polymer-based aqueous SEC (GFC) column OHpak SB-806 HQ and various detectors. Ultraviolet (UV) at 280 nm covers general culture-derived impurities, and fluorescence (Ex at 280 nm and Em at 348 nm) responds mainly to proteins via tryptophan residue fluorescence. In addition, MALS scattered light (LS) provides a highly sensitive response especially for large objects like nanoparticles. Moreover, MALS gives an estimate of the target RMS (root mean square) radius. The fraction consisted mainly EV was separated from many culture-derived impurities and was found around 8 min. While UV and fluorescence provide important insights into the progress and efficacy of the purification process and profiling of purified products, they are less sensitive to EVs mostly composed of lipid membranes and containing trace amounts of protein/nucleic acid cargo. Light scattering (LS) is an effective EV tracking method, especially in the early stages of purification. SB-806 HQ is a high-performance aqueous SEC (GFC) column suitable for bioproducts with a sufficient pore size to hold and separate EV-class nanoscale objects. Combined with a variety of detectors, a comprehensive analysis of the complex bio-nano target preparation process is achieved.
EV fraction preparation conditions (1) Concentration step: Commercially available centrifugal ultrafiltration membrane 100 kDa (40 times concentrated) (2) Affinity step: Commercial affinity purification kit (equivalent to 10-fold concentration), sample preparation by Showa Denko Materials Co., Ltd.
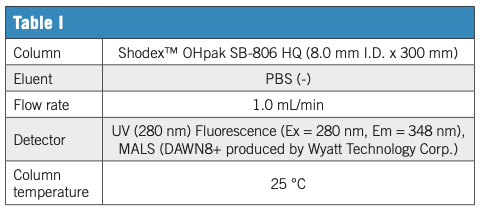
Figure 1: Sample 1: Cell culture supernatant 50 μL inj. 2: Concentrate 50 μL inj. 3: Crude product 15 μL inj. Process: supernatant concentrated by ultrafiltration; crude product obtained by affinity chromatography.
Reference
(1) J. Dai, Y. Su, S. Zhong, et al. Sig Transduct Target Ther 5, 145 (2020). https://doi.org/10.1038/s41392-020-00261-0

ShodexTM/Showa Denko America, Inc.
420 Lexington Avenue Suite 2335A, New York, NY, 10170
Tel. (212) 370-0033 x109
Website: www.shodexhplc.com
SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.