Application of Ambient Sampling Portable Mass Spectrometry Toward On-Site Screening of Clandestine Drug Operations
Special Issues
Worldwide trends in illicit drug use and production have shifted toward an increase in synthetic analogues and the emergence of new variations in their manufacture.
Worldwide trends in illicit drug use and production have shifted toward an increase in synthetic analogues and the emergence of new variations in their manufacture. Regulation of common precursors and existing psychoactive substances have led to modifications in clandestine synthetic pathways to use alternate starting materials and produce new structural variants that mimic previous illicit chemicals in physical and physiological properties, while not being officially scheduled through the U.S. Drug Enforcement Agency (DEA). The application of ambient sampling portable mass spectrometry (MS) systems to assist in combating clandestine drug operations represents a potential improvement toward on-site evidence screening over traditional colorimetric or solubility-based field kits, providing superior chemical discrimination with high accuracy, while simultaneously yielding information regarding cutting agents that may also be present. Coupling ambient ionization methods to these portable systems allows direct screening of potential evidence present in any state, from unknown white powders to the volatile solvents used during drug extraction and cleanup.
Recent trends in illicit drug usage have shown an increasing prevalence of synthetic analogs throughout the world. For example, seizures from clandestine methamphetamine synthesis operations in the United States quadrupled over a one year period from 2010 to 2011, and multiple countries throughout Europe have recently reported the seizure and dismantling of clandestine laboratories within their borders for the first time (1). In addition to traditional amphetamine-type stimulants, various new drugs of abuse and modifications of traditional illicit chemicals have gained prominence throughout the world, notably the class of synthetic cathinones marketed as bath salts or plant food (2). Many of the new psychoactives follow the same pattern of being labeled as "not for ingestion" to delay scheduling as controlled substances and to maximize the time they can be sold as "legal highs." After the drug has been scheduled, its usage can gradually decline as it is replaced by a new synthetic analog with a structure just dissimilar enough to circumvent current scheduling, yet possessing moieties that are mimetic in regards to physiological effects (1). Alternately, scheduling of commonly used precursors for target clandestine synthetic drugs is undertaken only to prompt the creation of new synthetic pathways that utilize legal or easily obtainable starting materials. For example, methamphetamine synthesis through reductive amination of phenyl-2-propanone has become more common with increasing controls on pseudoephedrine-containing pharmaceuticals because of legislation like the 2005 "Combat Methamphetamine Epidemic Act." Furthermore, the analysis of seized pills sold as ecstasy are frequently found to contain other illicit psychoactive substances with more readily available precursors (such as methamphetamine) in addition to 3,4-methylenedioxy-N-methylamphetamine (MDMA) (1).
The ongoing variability in illicit drug production represents a challenge to forensic analysis and investigation. Evidentiary analysis of controlled substances accounted for the second highest percentage (33%) of requests submitted to publicly funded forensic laboratories as of the 2009 Census of Publicly Funded Crime Labs, behind only forensic biology requests (34%). Additionally, requests for controlled substance analysis was the second highest evidence category in terms of the ever-growing backlog of casework, forming an estimated 12% of all backlogged samples across the nation (3). While many factors contribute to these backlogs, one major consideration is the large number of samples gathered during evidence collection and the lengthy sample preparation and analysis times required with current analytical methods. Accepted protocols for forensic laboratory analysis typically involve hyphenated mass spectrometry techniques, particularly gas chromatography–mass spectrometry (GC–MS) and liquid chromatography (LC)–MS, often requiring up to 30 min per chromatographic run in addition to the necessary sample preparation constraints (4).
On-site presumptive testing is commonly used not only to aid law enforcement officers in obtaining warrants, but also to prioritize samples that most likely contain chemicals of interest. This, in turn, allows increased efficiency by sending samples of higher relevance to off-site laboratories for confirmation. Because of their easily observable changes and simplistic procedures, colorimetric tests form the majority of presumptive tests (5), yet these field tests possess significant disadvantages. For example, the cobalt thiocyanate test for cocaine (commonly referred to as the Scott test) can produce target-specific color changes from other legal and innocuous substances, including lidocaine and diphenhydramine (found in over-the-counter allergy medicine) (6). Even with the inclusion of additional steps to the test procedure to increase specificity, it can still yield ambiguous results depending on the amount of powdered sample used, placing the burden of proper technique on end users. The Marquis test yields multiple color changes unique to different drugs of abuse, but results can be highly subjective, requiring the use of Munsell color diagrams to distinguish similar colors (7). Because of the higher likelihood of false positive or negative responses, a selection of multiple tests must be performed on each sample. Furthermore, tests may require hazardous reagents, such as concentrated acids that must be disposed of after testing, or unique reagents and special preparations for specific controlled substances (7,8).
Major gains in accuracy and precision toward unknown evidentiary analysis could be realized by incorporating portable analytical instrumentation, particularly MS systems (2,9–11) that are adaptable to newly developed ambient ionization methods (12–14) like desorption electrospray ionization (DESI-MS). This work describes the application of a portable ambient sampling MS system coupled with ambient ionization methods for use in the rapid screening of controlled substances and chemicals related to their manufacture. The sensitivity and specificity of MS coupled with the direct sample analysis afforded to DESI has high potential for usage in on-site forensic evidentiary analysis, providing significant advantages over traditional field testing kits while plausibly reducing the burden and backlog of today's forensic laboratory system.
Sample Preparation
Standard solutions of drugs of abuse and related compounds, including cocaine, MDMA, methamphetamine, and lidocaine, were prepared through serial dilution in methanol of chemical standards purchased from Cerilliant Corporation. Additionally, bulk diphenhydramine powder was purchased from Sigma Aldrich and dissolved in methanol to prepare standard solutions. Known masses of analyte were then deposited by spotting 1–10 μL aliquots of standard solutions upon surfaces of interest. Following complete evaporation of the solvent, residues were sampled for DESI-MS analysis using polyurethane foam transfer swabs purchased from Berkshire Corporation. An over-the-counter pharmaceutical containing pseudoephedrine was also purchased and ground into a powder that was subsequently swabbed and analyzed using DESI-MS without further preparation.
For transfer swab analysis, the swab was first lightly moistened with ~5 μL of methanol. Surfaces of interest were first swabbed along a horizontal axis in a zigzag motion while slowly rotating the swab back and forth to expose all of one face of the swab. The opposite face of the swab was then used to sample the surface along its vertical axis. Swabs were then immediately introduced to the DESI source using a positioning guide and analyzed with slow rotation of the swab to expose the entirety of the swab surface area to the DESI spray. For atmospheric pressure chemical ionization (APCI) studies, gaseous samples from solvents of interest (Sigma Aldrich) were collected from the headspace of storage containers containing the bulk liquids.
Instrumentation
All presented data was collected on a Griffin AI-MS 1.2 cylindrical ion trap mass spectrometer (FLIR Systems). The size (24 in. × 20 in. × 15 in., L × W × H), weight (98 lb), and ruggedness of this instrument makes it an amenable platform for field-based, crime scene investigation applications, and technical specifications have been reported elsewhere (2). DESI analyses were performed using the incorporated ESI-DESI ionization source without modification, which fixes the spray angle at 55° in respect to the sample or surface under investigation. Parameters used for DESI-MS included a spray solvent of 1:1 water–methanol with 0.1% (v/v) formic acid set to a 3-μL/min solvent flow rate, a spray voltage of 4 kV, and nebulizing gas (nitrogen) pressure of 100 psi. Spectra were recorded over an m/z 70–450 range, and tandem MS (MS-MS) data were collected on all analytes for identification purposes.
Additionally, a custom APCI source was used to enable the analysis of volatile solvents commonly employed in the extraction and drying steps of clandestine syntheses. The home-built source used a small diaphragm pump (KNF Neuberger) to pull ambient air in proximity to a sample of interest into the ionization source through a chemically inert perfluoroalkoxy alkanes [PFA] polymer sampling tube. The sampled air passes over a tungsten needle to which a 4-kV voltage is applied to generate a corona discharge toward the inlet capillary of the MS system. Gaseous analytes present in the sampled air are ionized via charge-exchange with naturally occurring reagent ions formed in the discharge region and transported into the MS inlet (15,16), while exhausted sample is expelled through the pump to allow continuous, stand-alone analysis.
End-User Methods
To provide an analytical technique amenable to field use by potentially nontechnical users, automated instrumental methods were created to detect and confirm chemicals of relevance to drugs of abuse and clandestine synthesis via MS and MS-MS spectral matching. Methods initially search for the presence of protonated ions corresponding to known analytes in the base MS scan. If criteria including a minimum number of scans observed and surpassed intensity threshold are met, the control software indicates a "warning" of a possible target substance and automatically performs the collection of MS-MS spectra for identification purposes; these criteria are optimized and stored in the software for each potential analyte. The collected MS-MS spectra are compared in real time to an on-board spectral library, "alarming" the verified presence of a target analyte based on known fragmentation patterns collected on analytical standards. After the prompted MS-MS spectral search, the instrument then reverts back to base MS scanning to detect any additional controlled substances or cutting agents that may be present within the sample, and this mode of operation continues for a preset time or until disrupted by the user. After sample screening is complete, users are presented with visual indications of any recorded warnings (yellow light) and alarms (red light) for target compounds. Of importance, at no time are end users required to perform spectral interpretation; all collected data are saved for chain of custody purposes and are instantly accessible.
Results and Discussion
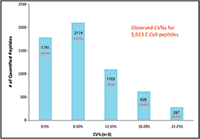
The proposed supplantation of current presumptive tests with MS-based portable instrumentation has the potential to solve the false positive or negative responses that currently hinder field determinations. As mentioned earlier, the Scott test for cocaine will signify the presence of cocaine even when compounds such as diphenhydramine and lidocaine are present. To demonstrate the capability of DESI-capable portable systems toward routine drug evidence identification, a mock sample consisting of 750 ng each of cocaine, diphenhydramine, and lidocaine was deposited as a residue on a glass substrate (19.4 cm2 total area). After physical contact with the slide, the transfer swab was placed into the DESI ionization source without further preparation, resulting in the spectra seen in Figure 1. Protonated molecules for cocaine, diphenhydramine, and lidocaine are clearly seen at m/z 304, 256, and 235, respectively, and all analytes were confirmed via MS-MS (not shown). Also seen is an in-source fragment of diphenhydramine, yielding a signature at m/z 167 stemming from the breakage of the ether linkage from the protonated precursor. In addition to being potential sources of false positives for the Scott test for cocaine, diphenhydramine and lidocaine may both be present in cocaine samples as cutting agents; for example, lidocaine is commonly used because of its ability to mimic the numbing properties of cocaine. This result also shows the power of the technique toward detecting and identifying adulterants besides the drug of abuse, information that can be helpful in determining the geographical origin of some seizures (17).
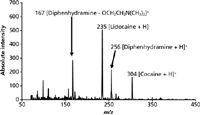
Figure 1: DESI-MS spectrum of a physical transfer swab used to sample a residue of 750 ng each of cocaine, diphenhydramine, and lidocaine swabbed off of a glass substrate.
The sensitivity afforded to the technique makes it well suited toward trace residue analysis, which could be advantageous in the assessment of past clandestine laboratory operations. Using the physical transfer swab method, residues from precursor chemicals and illicit products can be rapidly probed and detected. As seen in Table I, methamphetamine residues, seen in DESI-MS spectra as the protonated molecule at m/z 150, can be detected from a variety of surfaces including aluminum foil and PTFE-coated cookware at detection limits as low at 500 ng. Limits of detection (LODs) from surfaces are dramatically affected by the properties of the substrate. Relatively smooth surfaces like glass or nonstick cookware allow relatively low LODs to be obtained for drugs of abuse, while porous or geometrically complex surfaces can hinder physical transfer.
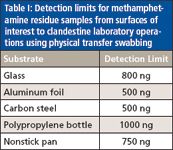
Table I: Detection limits for methamphetamine residue samples from surfaces of interest to clandestine laboratory operations using physical transfer swabbing
While trace analysis has benefits in forensic investigations, most routine evidence will consist of bulk samples (for example, an unknown "white powder"). To show applicability of the system to these types of evidence, powdered pharmaceutical tablets commonly used in clandestine syntheses were successfully investigated. Figure 2 shows the resultant DESI-MS spectrum from a swab used to sample roughly 0.250 g of a powdered Aleve Allergy and Sinus (Bayer Healthcare LLC) tablet, which contains 30 mg and 200 mg of the active ingredients pseudoephedrine and ibuprofen, respectively. After swab transfer, a gentle stream of canned air was then used to remove any loose powder from the swab to prevent clogging of the MS inlet capillary. The spectrum features peaks corresponding to the protonated molecules of both ibuprofen (m/z 207) and pseudoephedrine (m/z 166), as well as in-source fragment peaks at m/z 161 from loss of the carboxylic acid group of ibuprofen and at m/z 148 from the loss of a hydroxyl from pseudoephedrine. The inset in Figure 2 shows representative MS-MS spectra obtained on the MS system for protonated pseudoephedrine, yielding the majority fragment ion at m/z 148. Although care must be taken to avoid overloading the swab with powder and potentially blocking the inlet capillary, DESI analysis and the swabbing procedure was applicable to bulk powder analysis without preparatory work involving extraction and filtration. This technique can be extended to powdered drugs of abuse, as well, such as our recent work with seized synthetic cathinones (2).
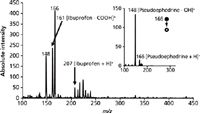
Figure 2: DESI-MS spectrum of an allergy and sinus pill containing ibuprofen and pseudoephedrine. MS-MS spectrum of pseudoephedrine (inset).
For a truly field-amenable technique, simplification must go beyond just the sampling step, particularly in regards to the user software interface. Figure 3 depicts screenshots of the developed "red light/green light" interface used to analyze a 200-ng residue of MDMA swabbed off of a glass substrate. During the ~2 min a user is prompted to present the swab sample, both MS (Figure 3, MDMA seen at m/z 194) and MS-MS (Figure 3b) data have been collected by the instrument and compared to the on-board spectral database. When both MS and MS-MS data match a compound of interest in the database, a color-coded prompt will "alarm" red for its confirmed presence, as seen in Figure 3c. During typical operation of the user interface, only the color-coded prompt is seen, but collected spectra are readily viewable if desired by an advanced operator.

Figure 3: Software screenshots from the analysis of MDMA using a simplified automated end user method: (a) MS spectrum and (b) MS2 spectrum from the DESI-MS analysis, showing the stored spectral data from the experiment; (c) visual identification screen presented to user after analysis, using "red light/green light" indications for simplification.
Although DESI-MS has the potential to screen solid and liquid forensic analytes with this portable system, it does not provide application to gaseous or highly volatile species. As several commercially available solvents are used for liquid–liquid extraction and drying steps in clandestine syntheses, the ability to identify these materials on-site could be of interest. To accomplish screening of volatile solvents with the portable system, a home-built APCI source was constructed and demonstrated. Figure 4a shows the resultant APCI-MS spectrum from the analysis of diethyl ether headspace, which was introduced into the source from the glass storage bottle by use of a sampling tube and external pumping. The simple spectrum collected shows protonated ethyl ether at m/z 75, as well as a protonated dimer at m/z 149 because of the relatively high gas concentration of the analyte. Figure 4b shows a representation of the constructed source, including the direction of gas flow and placement of corona discharge relative to the MS inlet. Spectra were obtained within seconds of collecting undiluted sample vapor, and signal response rapidly goes back to noise level after the sample tube is removed from the diethyl ether source, showing little to no carryover. The APCI source can be attached over the inlet capillary after the DESI source is lifted to its maintenance position, allowing quick switching of ionization sources in a "plug and play" manner to obtain information about solvents used during a clandestine synthesis; a photograph of the APCI coupling is shown in Figure 4c.
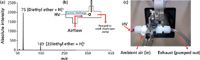
Figure 4: (a) Direct APCI-MS analysis of diethyl ether vapor, resulting in very simple spectra. (b) Diagram of the constructed APCI source, utilizing a corona discharge to produce analyte ions. (c) Photograph of the APCI source coupled to the MS system.
Conclusions
A portable, ambient sampling MS system coupled to DESI-MS was implemented for the screening of both residues and bulk samples of controlled substances and their synthetic precursors. Analysis was rapid and highly accurate when using MS-MS confirmation, surpassing that obtained with the current presumptive test. Additionally, DESI was capable of analyzing multiple types of drugs of abuse using a general-purpose spray solvent. Potential cutting agents and impurities from clandestine precursors were detected, suggesting the possibility of providing supplemental information on a controlled substance's origin or manufacturer. Pharmaceutical tablets and potential retail sources of a synthetic precursor for clandestine drug manufacturing were also successfully identified with no complication from the inactive ingredients and binders present. Automated methods were developed to enable simplified operation by nontechnical end users, using color-coded "red light/green light" indication of any target chemical present. Furthermore, a custom-built APCI source was constructed and demonstrated for the rapid identification of common solvents used in clandestine synthetic methods.
Acknowledgments
This project was supported by Award No. 2011-DN-BX-K552, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. MS-MS spectral assignments were made with assistance from high-resolution MS instrumentation acquired through support by the National Science Foundation MRI Program under Grant No. CHE 1337497. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the Department of Justice or the National Science Foundation.
Seth E. Hall and Christopher C. Mulligan are with the Department of Chemistry at Illinois State University in Normal, Illinois. Direct correspondence to: mulligan@ilstu.edu
References
(1) UNODC, World Drug Report 2013 (United Nations publication, Sales No. E.13.XI.6).
(2) K.E. Vircks and C.C. Mulligan, Rapid Commun. Mass Spectrom. 26, 2665–2672 (2012).
(3) M.R. Durose, Census of Publicly Funded Forensic Crime Laboratories, 2009. BJS Publication NCJ 238252; (Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice, U.S. Government Printing Office: Washington, DC, 2012).
(4) UNODC, Recommended Methods for the Identification and Analysis of Amphetamine, Methamphetamine, and Their Ring-Substituted Analogues in Seized Material (United Nations publication, Sales No. E.06.XI.1).
(5) Rapid Testing Methods of Drugs of Abuse Manual for Use by National Law Enforcement and Narcotics Laboratory Personnel (United Nations, New York 1994).
(6) Y. Tsumura, T. Mitome, and S. Kimoto, Forensic Sci. Int. 115, 158–164 (2005).
(7) C.L. O'Neal, D.J. Crouch, and A.A. Fatah, Forensic Sci. Int. 109, 189–201 (2000).
(8) J.A. Morris, J. Forensic Sci. 52, 84–87 (2007).
(9) C.C. Mulligan, N. Talaty, and R.G. Cooks, Chem. Commun. 1709–1711 (2006).
(10) J.M. Wells, M.J. Roth, A.D. Keil, J.W. Grossenbacher, D.R. Justes, G.E. Patterson, and D.J. Barket, Jr., J. Am. Soc. Mass. Spectrom. 19, 1419–1424 (2008).
(11) P.I. Hendricks, J.K. Dagleish, J.T. Shelley, M.A. Kirleis, M.T. McNicholas, L. Li, T.C. Chen, C.H. Chen, J.S. Duncan, F. Boudreau, R.J. Noll, J.P. Denton, T.A. Roach, Z. Ouyang, and R.G. Cooks, Anal. Chem. 86, 2900–2908 (2014).
(12) R.G. Cooks, Z. Ouyang, Z. Takats, and J.M. Wiseman, Science 311, 1566–1570 (2006).
(13) D.R. Ifa, A.U. Jackson, G. Paglia, and R.G. Cooks, Anal. Bioanal. Chem. 394, 1995–2008 (2009).
(14) R.M. Alberici, R.C. Simas, G.B. Sanvido, W. Romao, P.M. Lalli, M. Benassi, I.B.S. Cunha, and M.N. Eberlin, Anal. Bioanal. Chem. 398, 265–294 (2010).
(15) E.C. Horning, M.G. Horning, D.I. Carroll, I. Dzidic, and R.N. Stillwell, Anal. Chem. 45, 936–943 (1973).
(16) C.C Mulligan, D.R. Justes, R.J. Noll, N.L. Sanders, B.C. Laughlin, and R.G. Cooks, Analyst 131, 556–567 (2006).
(17) N. Stojanovska, M. Tahtouh, T. Kelly, A. Beavis, and S. Fu, Drug Test. Anal. 10.1002/dta.1684 (2014).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)