Analyzing Synthetic Cathinones Using Direct Sample Analysis Time-of-Flight Mass Spectrometry
Special Issues
Direct sample analysis coupled to high-resolution time-of-flight mass spectrometry (TOF-MS) may be effective at analyzing synthetic cathinones, especially for qualitative analysis, because it does not require potentially tedious sample preparation.
Direct sample analysis coupled to high-resolution time-of-flight mass spectrometry (TOF-MS) may be effective at analyzing synthetic cathinones, especially for qualitative analysis, because it does not require potentially tedious sample preparation. This technique will allow for a more rapid and simplistic analysis of synthetic drugs and increase laboratory throughput, as evidenced by the work of other researchers with different instrumentation for different compound classes.
Synthetic drugs known as "bath salts," "plant food," and "jewelry cleaner" have become prominent in the United States since their introduction in 2009 (1). Products exhibit colorful packaging labels that state "not for human consumption" to allow for legal possession and recreational use by circumventing control mechanisms (2). These drugs are sold extensively via online retailers and are also carried in "head shops" and independently owned convenience stores (3). The products generally contain cathinone derivatives and produce stimulant effects similar to methamphetamine and ecstasy (4). Cathinone is a schedule I drug under the Controlled Substances Act (CSA), but it has been modified to produce a wide variety of synthetic drugs. There are dozens of known cathinone derivatives on the market and hundreds more that are possible (5). In June of 2012 the United States Congress passed the Food and Drug Administration (FDA) Safety and Innovation Act that extended the CSA to include 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) as schedule I drugs (6).
Synthetic cathinones are sold as tablets, capsules, and powders and have been combined with other illicit controlled substances in previous cases (7). The abuse of these drugs has also led to deaths globally (8,9).
These products can be difficult to analyze because of the possible insolubility of adulterants, cutting agents, or other added materials. Generally, analysis requires some type of sample preparation to obtain the active compounds in a suitable form, such as an organic solvent solution. Direct sample analysis time-of-flight mass spectrometry (TOF-MS) may be effective, especially for qualitative analysis, because it does not require potentially tedious sample preparation. This technique allows for a more rapid and simplistic analysis of synthetic drugs and increases laboratory throughput, as evidenced by the work of other researchers with different instrumentation for different compound classes (10,11). Analysis of such designer drugs and the identification of individual compounds may help reduce their production and abuse.
Materials and Methods
Chemicals and Materials
Standard reference materials consisting of 3,4-methylenedioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone, 4-MMC), 3,4-methylenedioxy-N-methylcathinone (methylone), butylone, naphyrone, 3-fluoromethcathinone (3-FMC), 4-methylethcathinone (4-MEC), α-pyrrolidinopentiophenone (α-PVP), 3',4'-methylenedioxy-α-pyrrolidinobutiophenone (MDPBP), 3-methyl-α-pyrrolidino-propiophenone (3-MPPP), ethcathinone, and (S)-2-diphenylmethylpyrrolidine ([S]-desoxy-D2PM) were obtained from Cayman Chemical Company. Methanol (high performance liquid chromatography [HPLC]-grade) was purchased from Avantor. All solvents and chemicals used in the experiment are of a minimum purity of ACS reagent grade.
Samples
At various times during 2011, 14 samples were purchased at a local "head shop" in State College, Pennsylvania. The bath salts included "Recharge," "White Rush," "White Girls Bath Salt," "JET Premium Bath Salts," "Ocean Burst," "Ivory Wave," and "e Blast." All of the bath salts were in powder form except for "e Blast" which was in pill form. The sample "Cosmic Blast" was labeled as a "jewelry cleaner" and was in powder form. "Doves Original," "Doves Ultra," "Exotix Super Strong," "Dynamite NRG," "Diablo's XXX Extreme," and "Space Trips Ultra" were listed as "plant food" and were all in pill form.

Table I: Direct sample analysis TOF-MS instrumental conditions
Sample Preparation
Cathinone standards were prepared by placing less than 0.5 mg of solid sample on the mesh target screen and applying 5 μL of methanol. Because of incomplete solubility in methanol of the street materials, most likely from added inactive materials, the bath salt samples were directly sampled by placing them in glass sampling tubes.
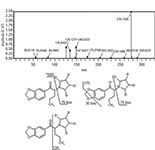
Figure 1: Mass spectra of standard reference material MDPV using CID fragmentation (upper panel). The parent ion is 276.1595 m/z and fragments include 205.0860 m/z, 149.0235 m/z, 135.0438 m/z, and 126.1274 m/z. Fragmentation pattern of MDPV determined from its mass spectrum (lower).
TOF-MS Instrumentation
An AxION DSA TOF-MS system (PerkinElmer) was operated under the conditions listed in Table I.
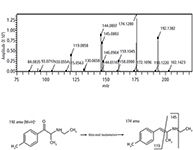
Figure 2: Mass spectrum of the reference standard 4-MEC (upper panel). The parent ion is at 192.1381 m/z and fragments include 174.1280 m/z, 145.0883 m/z, 119.0858 m/z, and 91.0563 m/z. Fragmentation pattern of 4-MEC determined by its mass spectrum (lower). The removal of water from 4-MEC is demonstrated. A double bond is formed between the carbonyl oxygen and the alpha carbon to form a hydroxyl group, which is removed to form water. Adapted from reference 12.
Results
Each standard was applied to the TOF-MS system's mesh target screen using 5 μL of methanol to retain the compounds. The mesh was then inserted into the system's sample tray and the standards were run as a sequence. Atmospheric positive chemical ionization (APCI) tune mix, or calibrant spray (Agilent Technologies), was run simultaneously with the standards to maintain mass accuracy. The lock mass ions used for this experiment were m/z 121.0509 and m/z 322.0481. Standards were analyzed using both normal MS analysis for determination of the accurate masses of the parent ions, and collision-induced dissociation (CID) for fragmentation purposes. The fragment ions were chosen based on the spectral data once background ions were accounted for (Figures 1 and 2). This was performed by subtracting the APCI tune mix from each standard spectrum.

Figure 3: Mass spectrum of "Ivory Wave" using CID fragmentation. The parent ion of MDPV (276.1596 m/z) is clearly present in the spectrum as well as fragment ions.
Particular substituted cathinone compounds were found to exhibit the loss of water because of formation of a double bond between the carbonyl oxygen and the alpha carbon to produce a hydroxyl group. This mechanism seems to be present in straight chained cathinone derivatives (12). Specifically, this occurs with 4-MMC, methylone, 3-FMC, butylone, ethcathinone, and 4-MEC for the standards that were analyzed in this experiment (Figure 2). The loss of water was not as evident in the compounds MDPV, MDPBP, 4-MPPP, naphyrone, and α-PVP.

Figure 4: Mass spectrum of "Ocean Burst" using CID fragmentation. The parent ion of butylone (222.1122 m/z) is clearly present. Fragment ions of butylone, ethcathinone, and 4-MEC are also indicated in the spectrum. Many of the fragment ions of ethcathinone and 4-MEC are of low intensity and are not immediately apparent. The fragment at 56.0130 m/z is most likely contributed by the sample matrix since it did not appear in the spectra of the reference standards.
The theoretical masses of the standard reference materials were compared to the measured masses obtained using direct sample analysis TOF-MS analysis. High mass accuracy (generally less than 5 ppm error) was demonstrated for the parent ions and the majority of the fragment ions (Table II).

Figure 5: Mass spectrum of "e Blast" using CID fragmentation. The parent ions of MDPBP (262.1436 m/z) and α-PVP (232.1694 m/z) are clearly present. Fragment ions of MDPBP, α-PVP, and 4-MEC are also indicated in the spectrum. The fragment at 56.0113 m/z is most likely contributed by the sample matrix since it did not appear in the spectra of the reference standards.
Bath salt samples were analyzed by adding a small portion of each product to individual glass sampling tubes. The tubes were then placed in a rack suitable for the direct sample analysis interface. The reagent gas ionized and fragmented the compounds without the need for sample preparation. Approximately 10 samples were analyzed without the use of CID. The parent ions were obtained and are listed in Table III. The parent ions and fragment ions were obtained for two random single component samples (Ivory Wave, Doves Ultra) and three multicomponent samples using CID (Table III). The remaining samples were analyzed without CID. These data are also listed in Table III. Again, the instrument showed high mass accuracy even for complex mixtures. The active components in the samples were identified by comparing the parent and fragment ions of the compounds found in the sample (Table III) to those of the reference materials (Table II).

Table II: Accurate masses of parent and fragment ions from standard reference materials
Conclusion
Using direct sample analysis TOF-MS, 14 samples were screened. Parent and fragment ions for 10 different cathinone compounds were detected with excellent mass accuracy of generally less than 5 ppm. The elimination of sample preparation makes analysis easier especially when tedious extraction methods or derivatization otherwise are used (13). This also significantly increases the number of samples that can be screened in a given time. Some current methods that use gas chromatography–mass spectrometry (GC–MS) can take up to 30 min to analyze one sample while the direct sample analysis TOF-MS approach enables the analysis of one sample in 15 s. The use of this approach could, therefore, significantly increase laboratory throughput for qualitative identification. The drawback with direct sample analysis TOF-MS, however, is that quantification of the active compounds cannot be performed. Additionally a library of standard reference materials is needed to identify the cathinones, which can be expensive or commercially unavailable. It is also difficult to identify particular isomers using this technique because of a lack of retention time data.
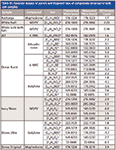
Table III: Accurate masses of parent and fragment ions of compounds detected in bath salt samples
Synthetic cathinones can be easily analyzed using direct sample analysis TOF-MS. Direct sample analysis TOF-MS has been shown to be an efficient analytical tool in the analysis of bath salt samples and can potentially replace current techniques to improve laboratory productivity. A similar approach may also be useful for additional compound classes where similar needs exist.

Frank Dorman is an associate professor at The Pennsylvania State University in University Park, Pennsylvania. Amanda Leffler is with the forensic science program at The Pennsylvania State University. Noelle Elliott and Carl Schwarz are with Perkin Elmer Corporation in Shelton, Connecticut. Direct correspondence to: fld3@psu.edu
References
(1) European Monitoring Centre for Drugs and Drug Addiction. Advisory Council on the Misuse of Drugs: Consideration of the Cathinones. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cathinones (accessed April 3, 2012).
(2) J. Fass, A. Fass, and A. Garcia, Ann. Pharmacother. 46, 436–441 (2012).
(3) H.A. Spiller et al., Clin Toxicol. 49, 499–505 (2011).
(4) M. Schechter et al., Pharmacol Biochem Be. 20, 181–184 (1984).
(5) National Alliance for Model State Drug Laws. Cathinone Derivatives Trade Name and Chemical Compound Chart. http://www.namsdl.org/documents/CathinoneDerivativesTradeNameandChemicalCompoundChart.pdf (accessed April 03, 2012).
(6) S. 3187:Food and Drug Administration Safety and Innovation Act. 112th Congress, 2011-2012. Passed June 27, 2012.
(7) Drug Enforcement Administration. Request for Information on Synthetic Cathinones. Microgram Bulletin. [Online] 2011, 44(4), 31-37. http://www.justice.gov/dea/programs/forensicsci/microgram/mg2011/mg0411.pdf (accessed April 3, 2012).
(8) P. Maskell et al., J. Anal. Tox. 35(3), 188–191 (2011).
(9) K. Lusthof et al., Forensic Sci. Int. 206(1–3), 93–95 (2011).
(10) R.B. Cody et al., Anal. Chem. 77(8), 2297–2302 (2011).
(11) R.G. Cooks et al., Science 311, 1566–1570 (2006).
(12) D. Zuba, Trends Anal. Chem. 32, 15–30 (2012).
(13) S.M. Wang et al., J. Chromatogr B. 825(1), 88–95 (2005).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







