Analytical Supercritical Fluid Extraction Goes Back to the Future
Past, present, and future developments of supercritical fluid extraction (SFE) are outlined. New vendor activity in terms of automation and hyphenation with chromatographic separations is discussed.
The past and future development of analytical supercritical fluid extraction (SFE) is traced in terms of experimental strategies, applications, vendor support, and timely acceptance of the existing technology. The current state of the art is compared with research activity in the 1980s. New vendor activity, in terms of automation and hyphenation with chromatographic separations, is discussed.
For someone who has between 1980 and 2000 (i) published hundreds of peer-reviewed manuscripts on analytical supercritical fluid extraction (SFE) for chromatographic analysis and isolation of additives, neutraceuticals, pollutants, and so on; (ii) co-taught short courses on SFE in Europe, South America, North America, and Asia; and (iii) guest lectured numerous times on an ACS short course entitled "Sample Preparation for Chromatography" organized by Harold McNair, the current state of SFE does sometimes sound as if we are going back to the future.
A sample preparation survey in 2002 suggested that SFE was being used by less than 2% of respondents. A similar survey repeated in 2012 showed that the use of SFE had only doubled, despite the obvious overall advantages of supercritical carbon dioxide-based fluids (1).
The situation now is different from the 1980s because instrumentation is more robust and engineering applications in food, pharmaceuticals, bioanalytical, and materials are more plentiful. On the other hand, analytical SFE has not changed very much because there is a lack of support from major vendors and newcomers to the technology are forced to re-learn SFE history and protocols that have been around for years.
To put it in perspective, consider a report of the 9th Annual Waste Testing and Quality Assurance Symposium held in Crystal City, Virginia (USA) dated 23 July 1993 by Robert Stevenson, entitled "SFE in Purgatory". He writes: "SFE generates high interest in surveys, but low sales. All the necessary groundwork seems to be there, but where are the orders? Where are the users?"
Parenthetically, at about this same time, it was reported by experts in the field that "SFE is over the hump and that it is rapidly developing into the extraction method of choice for the 21st century with more and more laboratories around the U.S. and the world embracing it for sample cleanup and sample preparation" (2). No doubt this assessment was partly based upon the fact that between 1987 and 1989 more than 100 papers were published concerning the use of supercritical fluids for extraction (3).
Possible explanations for the perceived current lack of enthusiastic growth among analytical SFE practitioners are the limited number of suppliers that market the technology today and the exodus of vital vendors such as Hewlett Packard, Suprex, Dionex, ISCO, and Lee Scientific from the field around the turn of the century. A somewhat similar vendor exodus occurred slightly earlier in time in the field of SFC but, thanks to the efforts of Terry Berger and associates, the drought was not as long-lived, and SFC has survived to "live" again. Hopefully many of the developments that benefited the SFC community will now foster further development of analytical SFE. Currently, there appears to be a small core of established vendors, as well as relatively new vendors, that are committed to making analytical SFE a viable method for sample preparation prior to chromatographic analysis. Today these vendors are Jasco, Inc., Supercritical Fluid Technologies, Inc., Waters Corp., Applied Separations, and Taiwan Supercritical Technology.
The problem of long-term analytical SFE exploitation may also be in part an education issue as evidenced by the failure of academicians to both learn, explore, and apply the technology in junior-senior chemistry laboratory courses at the university level even when new instrumentation is made available. Furthermore, the perceived remarkable speed and versatility of SFE often tempts beginners to look for shortcuts rather than to examine the application systematically (4). A hasty approach to method development often leads to unexpected problems and analytical SFE is a sophisticated technology that is best mastered by adhering to the rigour required when developing any analytical method. It should be remembered that while there has been and continues to be a plethora of applications in engineering, SFE was only developed as an analytical technique in the mid-1980s. To gain some appreciation for past developments (5–10), the reader is encouraged to read the numerous reviews that have appeared recently as well as in the older literature. Look for details concerning the pros and cons of: (a) On-line or off-line coupling with a variety of separation techniques; (b) dynamic versus static extraction protocols; (c) solid-phase or liquid-phase extract trapping; (d) modifier addition to the matrix versus modifier addition to the fluid; (e) adsorbent in the extraction thimble to retain unwanted compounds such as water; (f) experimental strategies for extracting analytes from solids, gels, creams, sludges, liquids, and so on; and (g) polar-modified, high density CO2.
The use of SFE in modern process engineering applications was initiated in Germany during the late 1960s. These early studies showed that SFE was a viable alternative to distillation and solvent extraction processes. Furthermore, it allowed the processing of substances whose extraction could be adversely affected by high temperatures and the presence of solvent residuals. SFE using CO2 is now an established industrial process for the production of high-value natural products such as hops, decaffeinated tea and coffee, herbs and spices, medicinal herbs, seeds, and marine oils. Further examples include extraction processes where an undesirable component is removed from the matrix, such as pesticides from medicinal herbs. In ancillary fashion, SFE processing continues to find applications as shown by the variety of lipophilic extracts available as commercial products such as polyunsaturated fatty acid esters derived from fish oils, neat and roasted sesame seed oil, cranberry seed-based oils, oils high in n-3 and n-6 fatty acid content, and pumpkin seed extracts coupled with more traditional SFE-derived products such as decaffeinated coffee (11).
The scope of this report is therefore limited to advances concerning analytical SFE. The goals of this article are to both describe the current state of the art in relation to new instrumentation and to address unique extraction strategies, theories, and applications. In addition, interesting hyphenations of analytical SFE with chromatography and spectroscopy that have been reported during the past three years, as well as new aids for achieving successful extraction with CO2 will be considered.
Vendor Activity
The development of second generation SFC instrumentation by several major vendors over the past four to five years introducing improvements to mobile phase delivery, reproducible modifier introduction, and greater analytical sensitivity appears to have facilitated analogous selective advances in SFE. While these developments are encouraging, they will not take away, for example, the critical need for quantitative trapping that must occur when modified fluids are used to extract polar analytes that have marginal solubility in pure CO2. Furthermore, a greater understanding of matrix effects and the availability of universal SFE methodologies that are not specifically matrix and analyte sensitive and dependent is also desired.
Waters Corp. introduced at Pittcon 2013 an accelerated analytical supercritical fluid extraction system that provides unattended operation for method development, multiple samples, and multiple collection vessels. The unit utilizes ChromScope software to achieve better selectivity and specificity with no organic solvent exposure and no disposal cost. The system accommodates 10 sample vessels and 12 collection vessels that can be the same or different in vessels that range in volume from 5-mL to 15-mL. The key parameters of CO2 pressure, vessel temperature, percent co-solvent, and use of dynamic and static conditions, as well as collecting extracts at individual user criteria can all be programmed and run unattended. The unit may use up to six different co-solvents to develop new methods for multiple samples while enhancing the extraction process.
A second product line is pre-assembled and cart-mounted that varies in size from 100 mL to 2 × 5 L. Based upon the control software chosen and the application-specific component options used, the system offers the ability to do everything from sophisticated studies to routine scale up. Complementary techniques such as counter current chromatography, particle formation (for example, RESS and SAS), phase monitoring, and reaction engineering options are available.
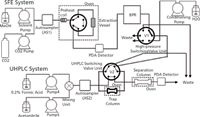
Two years earlier Jasco, Inc. introduced a supercritical fluid extraction coupled to ultrahigh-pressure liquid chromatography (SFE–UHPLC) system (Figure 1). The vendor noted that hyphenating multiple methods in an analysis system has many advantages such as decreasing the analysis time, reducing cost, simplifying sample pre-treatment, and increasing sensitivity and selectivity. In addition to hyphenation of SFE with SFC, gas chromatography (GC) and HPLC have been reported in the open literature (12). Hyphenation of SFE and UHPLC by Jasco, Inc. has only been reported in a vendor application note. The on-line SFE–UHPLC employs valve-switching techniques. Piperine in peppers was extracted effectively in the SFE system and then trapped and concentrated on the trap column that was then connected in the high-pressure switching valve unit section (13). After the extraction, a conditioning solvent was delivered from the conditioning pump to remove the gaseous CO2 in the trap column. The extract was then automatically introduced into the UHPLC system and subjected to rapid separation and photodiode array detection with high sensitivity. The overall system is reported to offer very high speed and extremely sensitive analysis without complicated pre-treatment. RSD for retention time was listed as 0.36% and for peak area was 1.91%. Overall piperine recoveries were 96.0%.
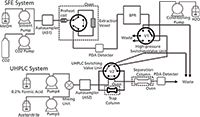
Figure 1: Schematic of a SFEâUHPLC system.
Several other vendors are quite active and supportive of advances in SFE. Applied Separations, Inc. offers a four vessel simultaneous oven-based extraction system with modifier addition capability. Temperatures up to 240 °C, pressures up to 680 bar, and flow rates up to 400 mL/min can be accommodated.
PIC Solution, Inc. has recently reported an useful technique to improve the quality of preparative separations called "injection by extraction" as a substitute for traditional mixed stream injection and modifier stream injection (14,15). Often one is able to load significantly more sample using this technique because of better solubility and less peak distortion. In addition, the vendor notes that the procedure avoids many of the problems associated with more conventional injection techniques. In particular sample precipitation, which is a problem often met in preparative SFC, is eliminated by dissolution of the sample in the mobile phase. The technique is also useful for purification of samples with insoluble impurities or that require extraction from a matrix before separation.
Hyphenation of SFE
Historically, the development of analytical SFE has been associated with a form of chromatography. The research of Stahl in Germany combined SFE with thin layer chromatography (16). In 1989, Anderson and colleagues reviewed some of the theoretical considerations surrounding the use of SFE as the means of introducing samples into chromatographic systems (17). The data presented indicated that SFE could be fine-tuned into a selective sample preparation or injection method for chromatography. The practice of analytical SFE is divided between "off-line" and "on-line" methods. Such definitions refer to the mechanism of conducting the extraction. The on-line methods are usually combinations of SFE with ancillary techniques such as GC, SFC, and HPLC. Off-line SFE offers more flexibility with respect to extracting different sample sizes and types, as well as in the choice of the final analytical method. The bottom line is that the selection of an analytical SFE method should be based on the problem faced. The design of the interface is a crucial aspect in developing a hyphenated technique involving the direct coupling of SFE to a destructive or non-destructive analytical detector.
More current studies concerning hyphenation continue to be published in the open literature. A direct aqueous SFE system designed to extract water samples contained in vials has been coupled with a reversed-phase LC–MS–MS system using a single 10-port valve (18). An SFE trap system using a C1 stationary phase connected to a C18 analytical HPLC column enabled the SFE–LC–MS–MS analysis of three polyether ionophore antibiotics in water. In another study, a new hyphenated technique for the extraction and determination of isoflavones in sea and freshwater algae and cyanobacteria with sonication sample pretreatment has been developed (19). A third reported study concerns the development of an analytical strategy based on rapid extraction techniques coupled to comprehensive bi-dimensional gas chromatography (GC×GC) for the characterization of the volatile fraction of tobaccos. The high peak capacity of GC×GC allowed global extraction techniques that do not focus on restricted chemical families to be considered (20). Finally, a novel, sensitive, and selective method for the separation and quantification of a group of nonpolar heterocyclic amines in commercial meat samples has been developed (21). The method is based on the combination of a SFE procedure followed by analysis of the extracted plug by capillary electrophoresis under fluorimetric detection.
Applications of SFE
The bioanalytical applications of supercritical fluid techniques such as SFE are of increasing interest. The main role of this technique is in the sample preparation and separation of biologically active compounds such as drugs and their metabolites, as well as endogenous compounds (22). The selection of the operating conditions depends on the specific compound or compound family to be extracted. Molecular weight and polarity have to be taken on a case-by-case basis. Some recently published applications have been selected to demonstrate the great variety of applications and to indicate the diversity of journals in which SFE is presented: (a) SFE of triterpenic acids from Eucalyptus globulus bark (23); (b) acrylamide from coffee (24); (c) monoterpenes from coniferous needles (25); (d) lycopene from tomatoes (26); (e) amphenicols from shrimp (27); (f) cannabinoids from marihuana (28); (g) abuse drugs in biological media (29); and (h) benign resolution of trans-1,2-cyclohexanediol by two-step SFE (30).
In summary, there is not only interest in SFE as an analytical tool but also for process development. It is clear from the recent literature that SFE for processing commands a greater interest at this time in a variety of areas such as food science, natural products, pharmaceuticals, bioprocessing (31), and environmental science. Advances in analytical SFE appear to have waned during the past decade as vendor commitment to the technology has been sporadic. Yet, mathematical modelling appears to be on the rise (32). Improved instrumentation for analytical applications such as automation, coupling to chromatographic and spectroscopic techniques, and sample throughput would benefit the technology. The time seems right for analytical SFE to expand on the gains made over the past 25 years as a rapid and efficient sample preparation tool. The technological developments first made in fast liquid chromatography and now supercritical fluid chromatography will no doubt bring greater emphasis and applications to analytical SFE.
Larry T. Taylor is Emeritus Professor of Chemistry at Virginia Tech, Blacksburg, Virginia, USA. Please direct correspondence to ltaylor@vt.edu
References
(1) R.E. Majors, LCGC (2002, 2012).
(2) C.L. Phelps, N.G. Smart, and C.M. Wai, J. Chem. Ed. 73(12), 1163–1168 (1996).
(3) M.S. Ray, Sep. Sci. Tech. 29, 2203 (1994).
(4) W. Pipkin, LCGC 10, 15 (1992).
(5) J.W. King and M.L. Hopper, J. AOAC International 75(3), 375–378 (1992).
(6) J.M. Levy, R.M. Ravey, and R. Panella, LCGC 15, 570 (1998).
(7) M.Herrero, J.A. Mendiola, A. Cifuentes, and E. Ibanez, J. Chromatogr. A 1217, 2495–2511 (2010).
(8) M.D. Luque de Castro, M. Valcarcel, M.T. Tena, Analytical Supercritical Fluid Extraction (Springer-Verlag, New York, USA, 1994).
(9) L.T. Taylor, Supercritical Fluid Extraction (John Wiley & Son, Inc, New York, USA, 1996).
(10) Analytical Supercritical Chromatography and Extraction, M.L. Lee and K.E. Markides, Eds (Chromatography Conferences, Inc, Provo, UT, USA, 1990).
(11) J.W. King and J.E. France, Basic Principles of Analytical Supercritical Fluid Extraction, in Analysis with Supercritical Fluids: Extraction and Chromatography, B. Wenclawiak, Ed (Springer, Berlin, Germany, 1992).
(12) Hyphenated Techniques in Supercritical Fluid Chromatography and Extraction, K. Jinno Ed (Elsevier, Amsterdam, the Netherlands, 1992).
(13) M. Saito et al., American Laboratory website, posted 1 February 2011 (http://www.jascoinc.com/blog/chromatography-applications/blog/2013/02/2/).
(14) M. Shaimi, "Direct Sample Injection Technique for Preparative Supercritical Fluid Chromatography: Solventless Injection," presented at PREP 2001, Washington, DC., USA.
(15) K. Gahm, "On-Line Coupling of Supercritical Fluid Extraction with Supercritical Fluid Chromatography for Chiral Separation of Racemic Mixture Containing Insoluble Impurities", presented at SFC 2013, Boston, MA, USA.
(16) E. Stahl, K.W. Quirin, and D. Gerard, Dense Gases for Extraction and Refining, (Springer-Verlag, Berlin, Germany, 1988).
(17) M.R. Andersen, J.T. Swanson, N.E. Porter, and B.E. Richter, J. Chromatogr. Sci. 27(7), 371–377 (1989).
(18) E.D. Ramsey, A.T. Rees, G. Wei, J.Y. Liu, and X.H. Wu, J. Chromatogr. A 1217, 3348–3356 (2010).
(19) B. Klejdus, L. Lojkova, M. Plaza, M. Snoblova, and D. Sterbova, J. Chromatogr. A 1217, 7956–7965 (2010).
(20) J. Vial, D. Thiébaut, P. Sassiat, M.S. Beldean-Galea, M.J. Gomez Ramos, G. Cognon, S. Mallipattu, B. Teillet, and M. Bouzige, J. Chromatogr. Sci. 48(4), 267–273 (2010).
(21) F. De Andres, M. Zougagh, G. Castaneda, and A. Rios, Electrophoresis 31(13), 2165–2173 (2010).
(22) A. Rios, M. Zougagh, and F. De Andres, Bioanalysis 2(1), 9–25 (2010).
(23) R.M.A. Domingues, M.M.R. de Melo, E.L.G. Oliveira, C.P. Neto, A.J.D. Silvestre, and C.M. Silva, J. Supercrit. Fluids 74, 105–114 (2013).
(24) M. Banchero, G.Pellegrino, and L. Manna, J. Food Eng. 115(3), 292–297 (2012).
(25) J. Fojtova, L. Lojkova, and V. Kuban, Cent. Eur. J. Chem.8, 409–418 (2010).
(26) B.A. Domadia and N.R. Vaghela, J. Chem. Pharm. Res. 5(4), 188–191 (2013).
(27) W.L. Liu, R.J. Lee, and M.R. Lee, Food Chem. 121(3), 797–802 (2010).
(28) J. Omar, M. Olivares, M. Alzaga, and N. Etxebarria, J. Sep. Sci. 36(8), 1397–13404 (2013).
(29) D.J.E. Mirson and O.E. Roses, Int. J. Environment and Health 4(1), 18–39 (2010).
(30) E. Szekely, G. Bansaghi, P. Thorey, P. Molnar, J. Madaarasz, L. Vida, and B. Simandi, Ind. Eng. Chem. Res. 49(19), 9349–9354 (2010).
(31) O. Catchpole, S. Tallon, P. Dyer, F. Montanes, T. Moreno, E. Vagi, W. Eltringham, and J. Billakanti, Amer. J. Biochem. Biotech. 8(4), 263–287 (2012).
(32) L.G. Oliverira, A.J.D. Silvestre, and C.M. Silva, Chem. Eng. Res. Design 89(7), 1104–1117 (2011).
(33) B. Honarvar, S.A. Sajadian, M. Khorram, and A. Samimi, J. Chem. Eng. 30(1), 159–166 (2013).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



