Analysis of Tobacco-Specific Nitrosamines in Cigarette Tobacco, Cigar Tobacco, and Smokeless Tobacco by Isotope Dilution LC–MS/MS
Tobacco-specific nitrosamines are important carcinogens in tobacco products. This article describes a fast, sensitive, selective, and robust method for analysis of these compounds in various tobacco samples by liquid chromatography–tandem mass spectrometry (LC–MS/MS). The separation of analytes from matrix interfering components was performed using a C18 column with simple LC–MS mobile phases. To minimize sample matrix effects on each analyte, a separate internal standard was used for each analyte. Two MS/MS ion pairs were used for each analyte for analyte confirmation and quantification, further enhancing the method’s selectivity and accuracy. The method was validated using different tobacco matrices.
Tobacco-specific nitrosamines (TSNA) are widely considered to be the most important carcinogens found in tobacco, smokeless tobacco, and tobacco smoke. Four of the most commonly studied TSNA compounds are N’-nitrosonornicotine (NNN), N’-nitrosoanatabine (NAT), N’-nitrosoanabasine (NAB), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). NAB is a weak carcinogen and NAT lacks activity, while NNK and NNN have been classified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer (1). The U.S. Food and Drug Administration (FDA) has included NNN and NNK in the established list as harmful and potentially harmful constituents in tobacco products and tobacco smoke (2). Tobacco product manufacturers must report their contents in tobacco products, according to FDA’s guidance (3).
Due to the health concerns and the recent progress made in reducing TSNA content in tobacco products, it is imperative to develop a fast, sensitive, selective, and robust analytical method that can determine low levels of TSNA in different tobacco products. Traditionally, the most widely used method for TSNA analysis has been gas chromatography (GC) coupled with a thermal energy analyzer (TEA) (4). However, the GC-TEA method has several drawbacks. First, although TEA is a nitroso-specific detector, it cannot differentiate between the coeluted nitroso-compounds. Therefore, it is less selective than a mass detector. Second, to attain good GC separation for the target compounds, extensive sample cleanup steps including liquid–liquid (L-L) extraction and solid phase extraction (SPE) are required. Third, analytes must be concentrated before injection to achieve good analyte signals due to the limited sensitivity of the method. Recently, LC–MS/MS methods have been developed for TSNA analysis, which provides greater sensitivity and selectivity over the GC-TEA method (5–7). However, one of the major concerns for the development and validation of a LC– MS/MS method is the sample matrix effects, which can lead to poor analyte recoveries, and decrease the method’s accuracy and precision (8). One way to reduce these matrix effects is to perform sample cleanup, such as L-L extraction and SPE, as shown in one of the LC–MS/MS methods for TSNA (5). However, this will increase the analysis time and cost, as well as potentially introduce variations in sample preparation. Another effective way to compensate for the matrix effects is to use stable isotope-labeled internal standards in all standards and samples. This can maintain high-throughput sample analysis without the need for sample cleanup. In addition to compensating for the sample matrix effects, the use of individual internal standards in a method is also helpful for reducing the variations during sample preparation and analytical procedures. However, one of the previously developed LC–MS/MS methods (6) used only two isotope-labeled compounds (NNN-d4 and NNK-d4) as internal standards for all the four analytes (NNN, NNK, NAT, and NAB). This could result in errors in the determination of NAT and NAB due to the significant analyte-dependent matrix effects found in TSNA analysis (7). To achieve accurate results in complex tobacco matrices, the author had developed an improved LC–MS/MS method for TSNA analysis in cigarette tobacco and tobacco smoke using four isotope-labeled internal standards to match the four TSNA analytes (7). In this study, to provide better analyte confirmation and quantification in various tobacco matrices, multiple MS/MS transition ion pairs were investigated for each analyte, and at least two MS/MS transition ion pairs were used in the final method for each analyte, which further enhanced the method’s selectivity, accuracy, and robustness. Sample preparations involved only a simple analyte extraction with aqueous buffer, followed by filtration using a syringe filter. These improvements allowed us to take full advantage of the fast analysis, great sensitivity, and high selectivity of LC–MS/MS method without the need of extensive sample cleanup procedures. The method was validated using different tobacco sample matrices and thus its applications were extended to the determination of TSNA in a wide range of tobacco products, including cigarette tobacco, cigar tobacco, and smokeless tobacco samples.
Experimental
Standards and Sample Preparation
Certified reference standards (1 mg/ mL for each TSNA, Sigma-Aldrich) and the deuterium labeled internal standards (Toronto Research Chemicals) were used for the preparation of calibration standards and samples after dissolving in acetonitrile and appropriate dilutions. The Kentucky Reference cigarette tobacco (KY3R4F, obtained from the Tobacco and Health Research Institute, University of Kentucky), the CORESTA recommended reference smokeless tobacco products (CRP, offered by Department of Crop Science, North Carolina State University), and some commercial cigarettes and mini cigars (purchased from a local store in Toronto, Canada) were analyzed for TSNA contents in the samples. Two batches of CRP reference samples were obtained. One batch of samples (produced in 2009) were labeled as: CRP1 (snus), CRP2 (moist snuff), CRP3 (dry snuff), and CRP4 (chewing tobacco). Another batch of samples (produced in 2016) were labeled as: CRP1.1 (snus), CRP2.1 (moist snuff), CRP3.1 (dry snuff), and CRP4.1 (chewing tobacco).
A mixed TSNA standard solution (10 µg/mL of each analyte) and a mixed internal standard (IS) solution (10 µg/mL of each standard) were prepared separately by diluting their primary standard solutions with methanol. A secondary mixed standard solution (1.0 µg/mL of each analyte) was prepared by diluting the above mixed standard solution with 50% methanol solution (in water, v/v). A secondary mixed IS solution (200 ng/mL of each) was prepared by diluting the above mixed IS solution with 50% methanol solution (in water, v/v). Ten levels of calibration standards, containing each TSNA at 0.01, 0.02, 0.05, 0.1, 0.5, 1, 5, 10, 50, and 100 ng/ mL, were prepared from the secondary mixed standard solution by dilutions with reagent water. Each calibration standard contains 20 ng/mL of each IS.
A 1.0 g of ground and homogeneous tobacco sample was spiked with 60 µL of the IS solution (10 µg/mL), extracted with 30 mL of 100 mM ammonium acetate solution in a 50 mL centrifuge tube, and agitated for 40 min on a shaker. Each sample contained 20 ng/ml of each IS. The sample solution was then centrifuged and filtered through a syringe filter (if necessary), and analyzed directly by the LC–MS/MS method without further treatment. The extracted samples that exceed the calibration range for any TSNA (such as CRP3, CRP3.1, CRP4.1, and cigar filler sample S2 in this study) were further diluted with extraction solution, and reanalyzed. Since TSNAs are light sensitive, all standards and samples were prepared in amber or light-protected glassware, and stored in a freezer.
Quality Control Sample Preparation
To test possible interference and contamination from reagents or materials used and from the sample preparation processes, a laboratory reagent blank (LRB) was prepared per work shift. The values of LRB should be close to zero, or at least less than the LOQ of the method. Otherwise, an investigation on the source of contamination must be carried out.
An LRB sample was prepared by following the same procedures as for tobacco sample preparation described above, using 0.0 gram of tobacco sample. To study possible analyte loss and contamination during sample preparations, a laboratory fortified blank (LFB) sample was prepared per work shift. An LFB sample was prepared by following the same tobacco sample preparation procedures, using 0.0 gram of tobacco sample spiked with a known amount of analyte solution.
During method validation, two LFB samples were prepared by spiking the analyte in two different concentrations (10 ng/mL for LFB1 and 100 ng/mL for LFB2). To evaluate sample matrix effects and analyte recovery from tobacco sample matrix, a laboratory fortified matrix sample (LFM) was prepared per work shift. An LFM sample was prepared by following the same tobacco sample preparation procedures, using 1.0 gram of a tobacco sample spiked with a known amount of analyte. The percent recovery is calculated by comparing the difference of the spiked (LFM) and nonspiked sample results and the expected (spiked) value. During method validation, three different tobacco sample matrices (cigarette tobacco, cigar tobacco, and smokeless tobacco sample, respectively, representing all the tested tobacco sample matrices) were evaluated, and two LFM samples were prepared for each tobacco matrix by spiking two different concentrations of analyte (10 ng/mL for LFM1 and 100 ng/mL for LFM2, corresponding to 300 and 3000 ng TSNA/g tobacco), and thus in total, six LFM samples were prepared. CORESTA-recommended reference smokeless tobacco samples CRP1, CRP2, CRP3, and CRP4 were also prepared, and the results obtained by this method were compared with those obtained from the CORESTA inter-laboratory studies (9).
Analytical Conditions
Analyte separation was performed using a UHPLC system (PerkinElmer LX-50) with a C18 column (Brownlee SPP, 2.7µm, 100 mm × 2.1 mm) and a simple mobile phase composition of water and methanol (flow rate at 0.35 mL/min). A10 µL of sample was injected and the total LC running time is seven min. Analytes detection was achieved using a triple quadrupole mass detector (PerkinElmer QSight 220) under positive ESI mode. Multiple MRM transition ion pairs were monitored for each TSNA and the IS to evaluate potential interfering components for any transitions in the sample matrices. The ion source conditions are: ESI voltage, 5800 V; drying gas, 120; nebulizer gas, 280; source temperature, 400 ºC and HSID temperature, 200 ºC.
Results and Discussion
LC–MS/MS Method Optimization
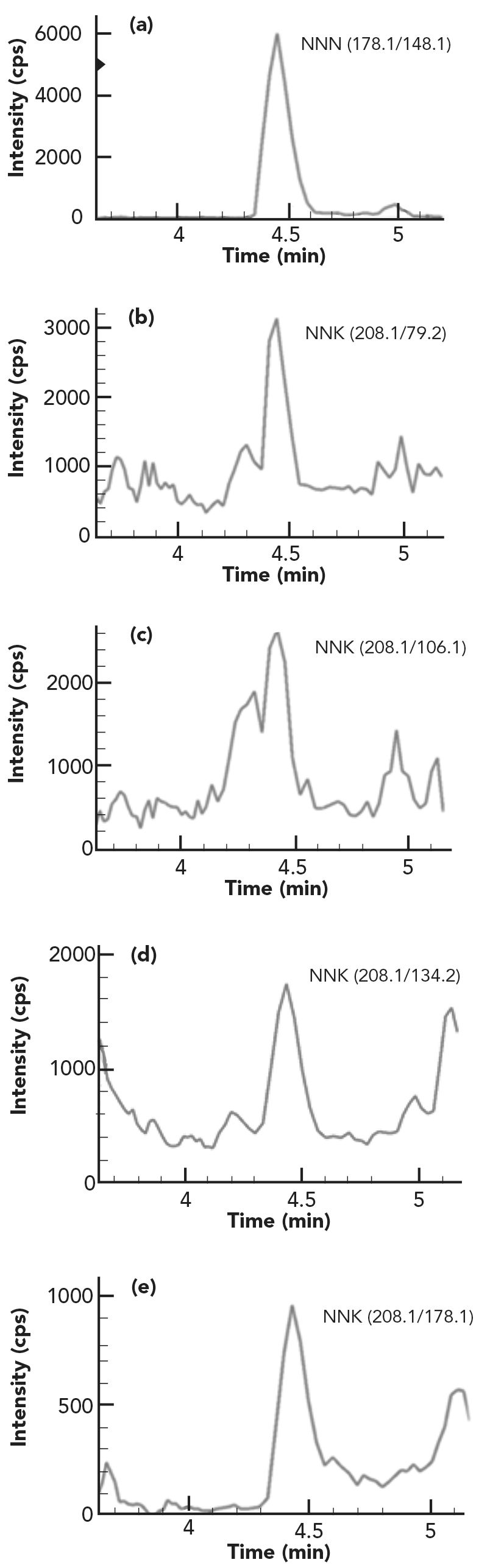
FIGURE 1: MRM chromatograms of (a) NNN, (b) NNK, (c) NAT, and (d) NAB in two calibration standard solutions: Std 1 (in red) contains each TSNA 0.01 ng/mL, Std 2 (in green) contains each TSNA 0.02 ng/mL, and laboratory reagent blank (LRB) is in blue.
To separate TSNA from interfering components in different tobacco sample matrices, several reversed-phase LC columns available in the laboratory were evaluated. As illustrated in Figure 1, good resolution for each TSNA peak was achieved using a C18 column (Brownlee SPP, 2.1 mm x 100 mm, 2.7-µm). More importantly, an impurity (unknown) peak in the calibration standards was well separated from the NAB peak, which would otherwise lead to a higher NAB slope and lower NAB results in samples if it was not separated from NAB peak. Mobile phase compositions of methanol:water and acetonitrile:water with or without acid or ammonium acetate were examined, and it was found that methanol:water without any additive provided higher analyte signals than acetonitrile:water compositions. Formic acid or acetic acid when used as the additives in mobile phases not only caused peak splitting for all TSNA analytes, but also led to reduced analyte signal. Adding ammonium acetate in mobile phases also decreased the analyte signal intensity due to ion suppression effects. Therefore, a simple mobile phase composition of methanol and water was used for final application. To detect TSNA compounds and their ISs, several product ions were generated at certain collision energies, and at least five MRM transitions were formed (data not shown but available upon request). The optimized MRM parameters for the final selected quantifier and qualifier ions were listed in Table I. Good signal intensity was obtained even at 0.01 ng/mL (ppb) for each analyte as shown in Figure 1, demonstrating superior sensitivity of the method for testing these important carcinogens.
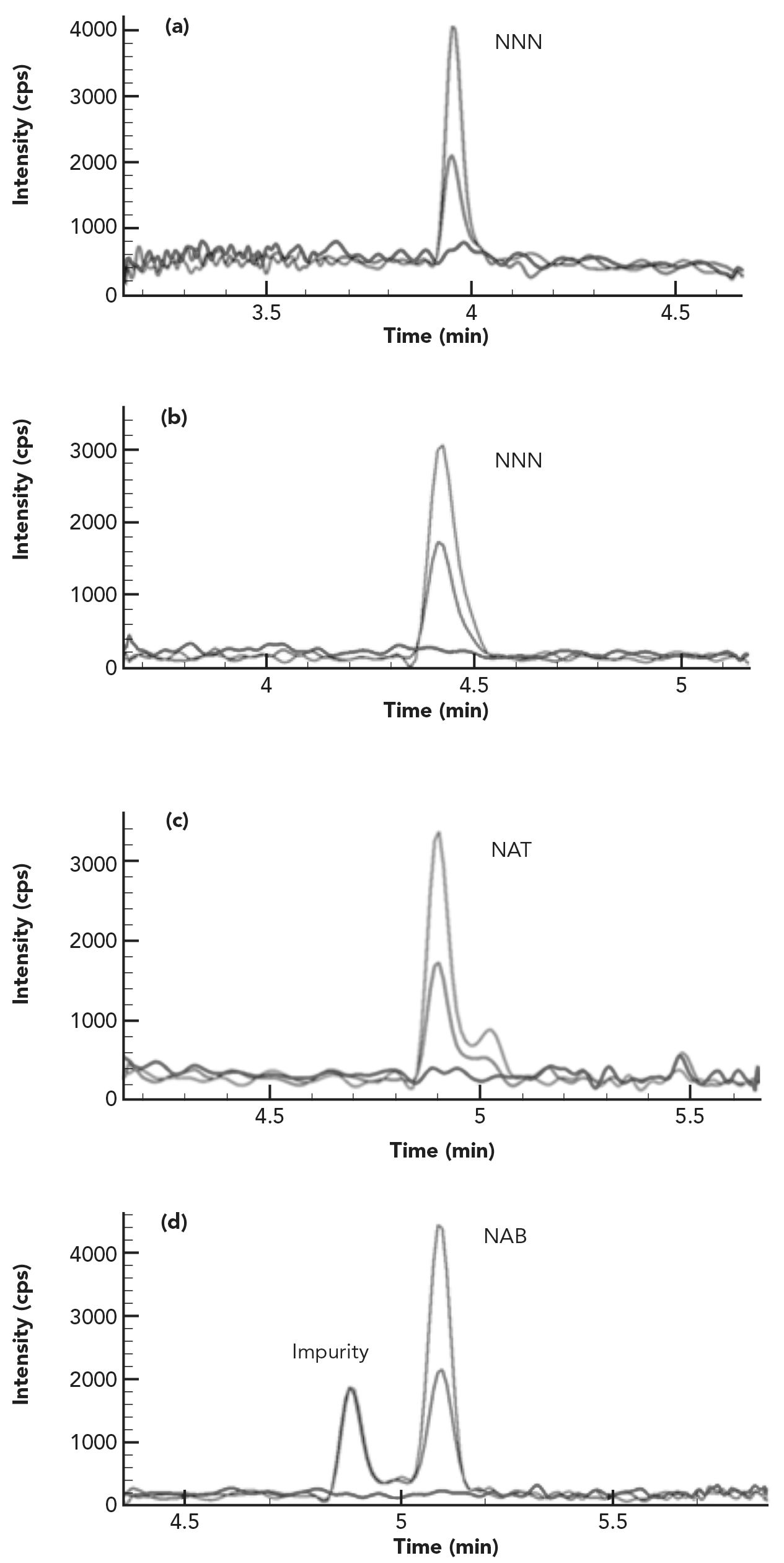
Sample Matrix Effects and Method’s Selectivity
Complex sample matrices, such as various tobacco products, can significantly affect the data quality and method’s selectivity, sensitivity, and accuracy. Therefore, sample matrix effects have received great attention in LC–MS/MS method development and validations. To reduce these effects, several approaches have been applied to LC–MS/ MS method development, such as sample clean-up, matrix-matched calibration method, isotope dilution with isotopically labeled compound as internal standard, the use of high efficiency UHPLC columns for better separation, and the use of alternative ionization sources (8). In a previous publication (7), The matrix effects in tobacco and tobacco smoke samples were studied, and it was found that sample matrix effects are both analyte dependent and sample matrix dependent. These results on tobacco matrix effects were further confirmed in this study by spiking the same concentration of IS solution into a calibration standard (neat solution without tobacco) and six different types of tobacco matrices, and comparing the signal intensity of each IS in tobacco samples with those in the standard solution. As demonstrated in Table II, the peak areas of each IS in different tobacco samples were reduced significantly compared to those in a standard, indicating severe ion suppression effects in tobacco matrices. In addition, these ion suppression effects were different for each analyte, and were much heavier in smokeless tobacco samples than in cigarette and cigar tobacco products. Thus, for TSNA analysis in complex tobacco sample matrices, a separate IS for each analyte that is required to effectively minimize or compensate for matrix effects on each analyte.
The method’s selectivity and analyte confirmation from tobacco samples can be evaluated by comparing the analyte retention time and mass spectrum information between a reference standard and tobacco samples. According to the regulatory guidance on analytical method validation, at least two MRM transitions should be used in a LC–MS/MS method (10,11). In this study, five MRM ion pairs were evaluated for each analyte in all sample matrices, and the final quantifier and qualifier ion pairs were determined based on the signal intensity, matrix effects, and interfering components among the five ion pairs examined. Due to severe tobacco matrix effects, some MRM ion pairs in a sample could suffer more from ion suppression effects and have more matrix interfering components than other MRM ion pairs.
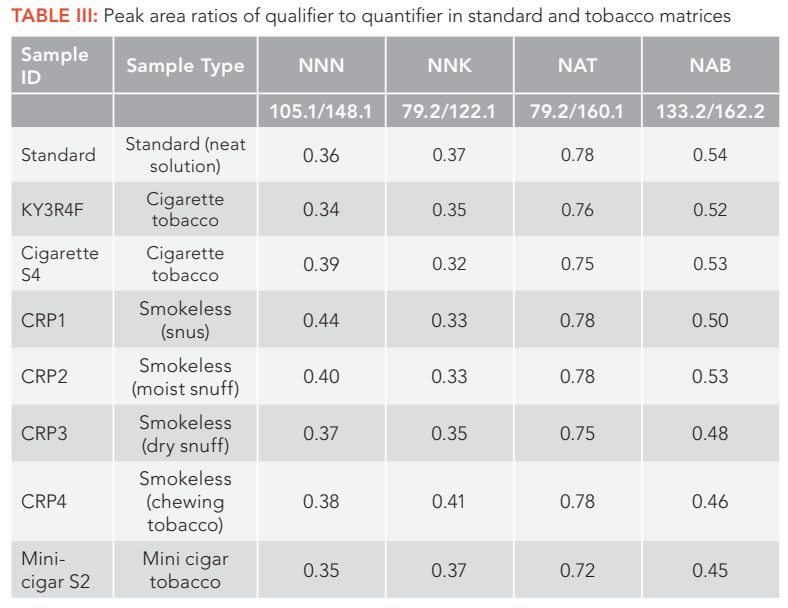
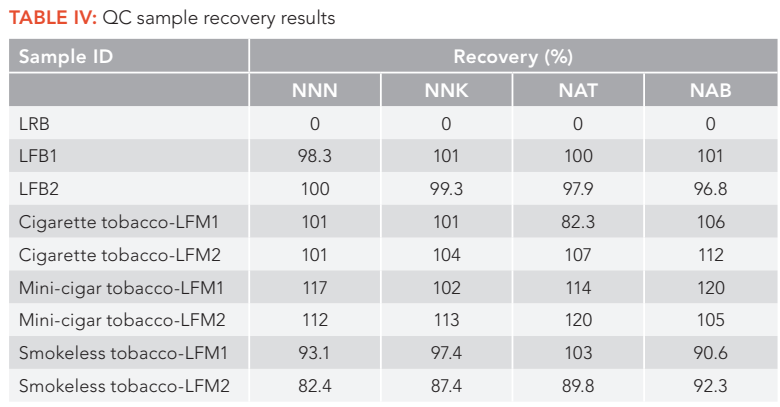
For example, for smokeless tobacco sample CRP1, the second MRM ion pairs of NNN suffered from heavy ion suppression effects, and had large matrix peaks on the right of the analyte peak as shown in Figure 2b. Also, the third MRM ion pairs of NNK had a big interfering peak at the left shoulder of the analyte peak as illustrated in Figure 3c. Thus, these MRM ion pairs should not be used for analyte confirmation and quantification. In addition, because different tobacco samples have different matrix effects for each analyte and the matrix effects are analyte dependent, it is recommended to evaluate all the MRM ion pairs of each analyte in all sample matrices before selecting the MRM ion pairs as a quantifier and a qualifier for final method and applications. Table I lists the final MRM ion pairs used for TSNA quantification (quantifier) and confirmation (qualifiers) in the method, selected after carefully investigating all the MRM ion pairs in all tobacco matrices studied. The peak area ratios of qualifier to quantifier ions for each analyte in all samples are consistent with those of a reference standard with variations less than 15% (as shown in Table III), confirming good purity of TSNA peaks and great selectivity of the method for analysis of TSNA in various tobacco matrices. These results demonstrated the advantages of using multiple MRM transition ion pairs for each analyte in LC–MS/MS method development, which could improve compounds confirmation, achieve better selectivity and obtain more accurate results.
FIGURE 2: The (a–c) three MRM chromatograms of NNN in smokeless tobacco CRP1.
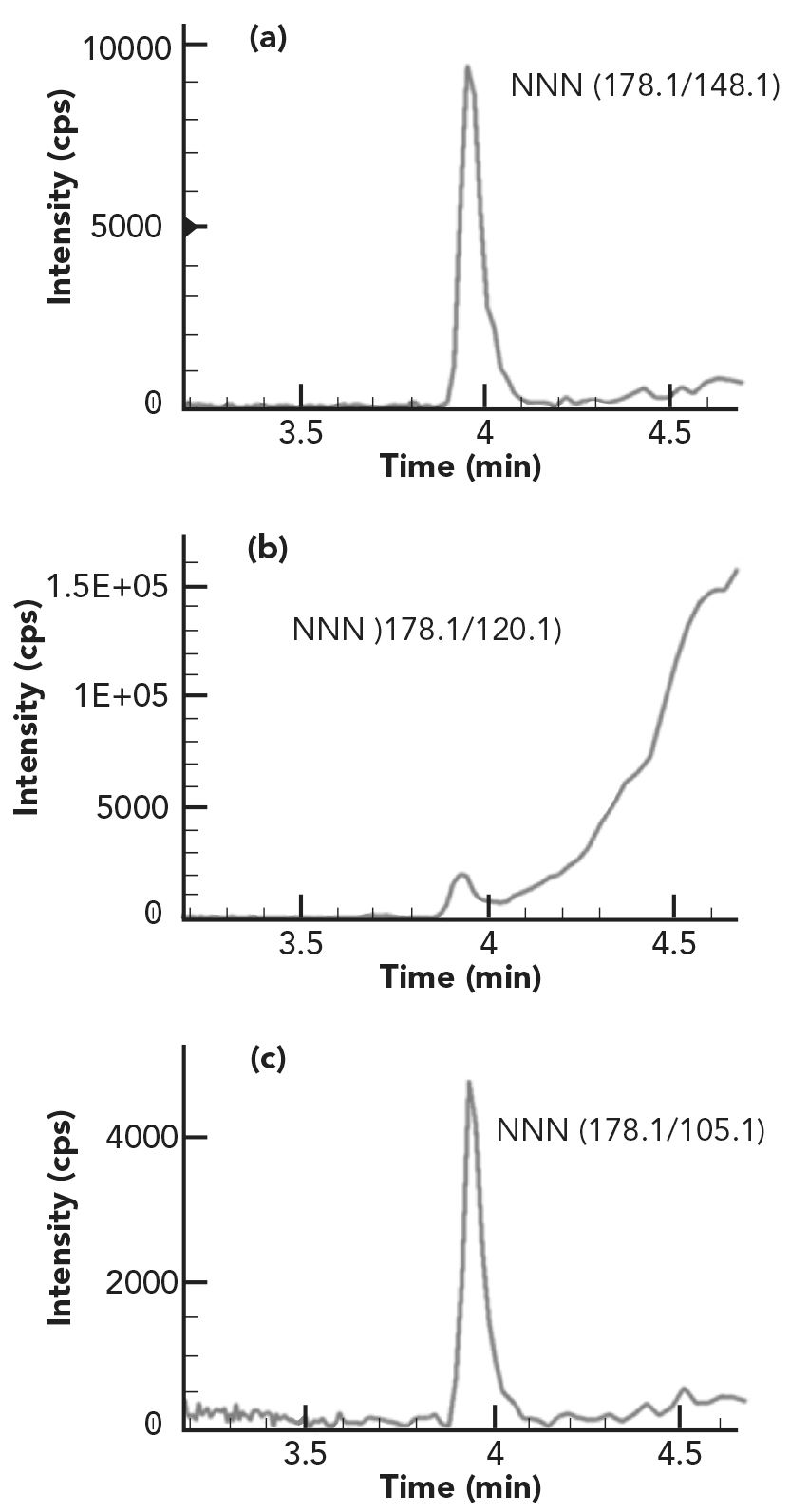
FIGURE 3: The (a–e) five MRM chromatograms of NNK in smokeless tobacco sample CRP1.
Method Validation
All calibration curves ranging from 0.01 to 100 ng/mL showed good linearity with correlation coefficients (R2) greater than 0.99. The method’s limit of detection (LOD) and limit of quantification (LOQ) were determined based on signal to noise ratio (S/N=3 for LOD and S/N=10 for LOQ) of the quantifier ion peaks in sample matrices. Although very low LOD (0.007 ng/mL) and LOQ (0.01ng/mL) can be obtained for each analyte in pure standard samples, the LOD and LOQ for real tobacco matrices are relatively higher due to heavy matrix suppression effects (as shown in Table II), especially for smokeless tobacco samples. Since matrix effects on each analyte are different in different sample matrices, the LOD and LOQ values of the method are different for different sample matrices. In other words, the LODs and LOQs of the method are sample matrix dependent and analyte dependent. The estimated LODs and LOQs of the method for TSNA in cigarette and cigar tobaccos are from 0.5 to 1.0 ng/g and from 0.7 to 3 ng/g, respectively, while the estimated LODs and LOQs for TSNA in smokeless tobaccos are from 1 to 5 ng/g and from 3 to 8 ng/g, respectively.
As shown in Table IV, no interference or contamination from reagents or glassware was observed in this study as demonstrated by the LRB sample results. Good recoveries were obtained for LFB samples, indicating no analyte loss or contamination during sample preparations. Method precision was assessed based on replicate analyses of a middle level standard (seven replicates) and the tobacco samples (three replicates). The precision was then calculated based on the percent relative standard deviation (%RSD) of the collected data. The %RSDs were 3.1 to 6.8% for TSNA in the standard, and 5.3 to 14.3% for TSNA in the tobacco samples, respectively. Method accuracy assesses how close the experimental value is to the expected value. Method accuracy was evaluated by the recovery of a known amount of analyte spiked to a tobacco sample (LFM samples). As shown in Table IV, the recoveries of analytes from the spiked samples were between 82.3 and 120%, demonstrating good accuracy of the method.
Method robustness was studied by slightly changing the experimental parameters such as the ammonium acetate concentrations in extraction solution (50, 100, and 200 mM, respectively), mobile phase gradients, and HPLC columns from a different batch. No significant differences in method’s performance and analyte results were observed and thus confirmed the robustness of the method. In addition, the method was validated on two LC–MS/MS systems with equivalent results obtained. The accuracy and robustness of the method were also evaluated using the certified reference smokeless tobacco products recommended by CORESTA. As illustrated in Table V, the TSNA results obtained by this method are in line with those obtained by inter-laboratory collaborative study (9).
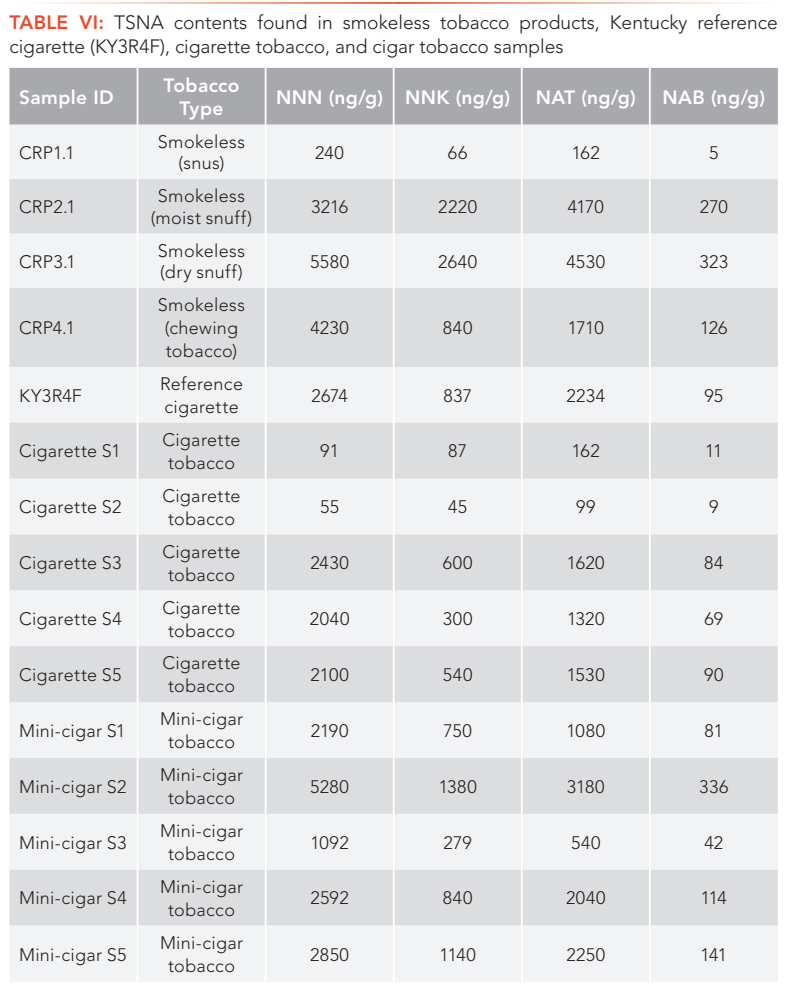
The method was applied to the determination of four important TSNA compounds in various tobacco products, including cigarette tobacco, cigar tobacco and smokeless tobacco samples as shown in Tables V and VI.
Conclusions
This study demonstrates a simple, fast, sensitive, selective, and robust method for the analysis of four important TSNA in various tobacco products. The method was validated using different tobacco matrices and was applied to TSNA determination in cigarette tobacco, mini-cigar tobacco, and smokeless tobacco samples with good linearity, precision, and accuracy. In comparison with the GC-TEA method, this isotope dilution LC–MS/MS method is simpler, faster, and without the need for sample cleanup and analyte concentration. This method can be easily extended to the analysis of TSNAs in other sample matrices, such as tobacco smoke and electronic cigarette (E-Cigs) samples.
Acknowledgements
The author wishes to thank Department of Crop Science, North Carolina State University (Raleigh, NC) for providing all the smokeless reference tobacco products free of charge.
Note: CORESTA (Cooperation Centre for Scientific Research Relative to Tobacco) is an association founded in 1956, the purpose being to promote international cooperation in scientific research relative to tobacco and its derived products.
References
(1) International Agency for Research on Cancer (IARC), IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Smokeless Tobacco and Some Tobacco Specific Nitrosamines. Lyon, France, 89, 1–592 (2007).
(2) US Food and Drug Administration, Harmful and Potentially Harmful Constituents (HPHCs) in Tobacco Products and Tobacco Smoke: Established list (FDA, Rockville, MD, 2012). https://www.fda.gov/tobacco-products/ rules-regulations-and-guidance/harmfuland-potentially-harmful-constituentstobacco-products-and-tobacco-smokeestablished-list.
(3) US Food and Drug Administration, Guidance for Industry Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke Under Section 904(a)(3) of the Federal Food, Drug, and Cosmetic Act (FDA, Rockville, MD, 2020). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/ reporting-harmful-and-potentially-harmful-constituents-tobacco-products-andtobacco-smoke-under.
(4) J. D. Adams, K. D. Brunnemann, and D. J. Hoffmann, J. Chromatogr., A 256, 347–351 (1983).
(5) W. Wu, D. L. Ashley, C. H. Watson, Anal. Chem. 75, 4827–4832 (2003).
(6) K. A. Wagner, N. H. Finkel, J. E. Fossett, I. G. Gillman, Anal. Chem. 77, 1001–1006 (2005).
(7) J. Wu, P. Joza, M. Sharifi, W. S. Rickert, J. H. Lauterbach, Anal. Chem. 80, 1341–1345 (2008).
(8) A. J. Krynitsky, J. W. Wong, K. Zhang, H. Safarpour, LCGC North America 35(7), 444–451 (2017).
(9) K. Wagner, J. Bunch, and M. Morton, CORESTA Smokeless Tobacco Sub-Group Technical Report; Technical Report for CORESTA Reference Products: Paris, France, December 2015; 1-48. https:// www.coresta.org/sites/default/files/technical_documents/main/STS-CTR_2015-CRP2015AnalysisWG4_Dec20152.pdf.
(10) US Food and Drug Administration, “Bioanalytical Method Validation Guidance for Industry,” proposed, Federal Register, 83(99), 24 May 2018, pp. 1–41. https:// www.fda.gov/downloads/drugs/guidances/ ucm070107.Pdf.
(11) European Commission Decision (2002/657/ EC) of 12 August 2002 concerning the performance of analytical methods and the interpretation of results, Off. J. Eur. Communities. L221, 8–36 (2002).
Jingcun Wu is with PerkinElmer Health Sciences Canada, in Woodbridge, Ontario, Canada. Direct correspondence to: jingcun.wu@perkinelmer.com

Advances in Non-Targeted Analysis for PFAS in Environmental Matrices
March 27th 2025David Megson from Manchester Metropolitan University in Manchester, UK, spoke to LCGC International about the latest developments in non-targeted analysis (NTA) of per- and polyfluoroalkyl substances (PFAS) in environmental matrices based on a recent systematic review paper he has collaboratively published (1).
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.








