The Why, What, and How of Headspace GC
The first question to ask is, why would headspace GC be a good sampling technique?
Question: Why would headspace GC be a good sampling technique?
Answer: Headspace sampling is excellent for the qualitative or quantitative analysis of volatile species in samples that can be efficiently partitioned into the headspace gas volume from either a liquid or solid matrix. It is also a good technique for the analysis of samples where the entire sample should not be injected into the GC instrument (i.e. dirty samples). Headspace sampling is also particularly amenable to the analysis of trace levels of analytes.
Typical examples of headspace analyses include:
- Volatile Organic Compounds (VOC) from wastewater and contaminated land samples
- Residual solvents in packaging and pharmaceuticals
- Blood alcohol and toxicology screening
- Aroma components from food and beverages
- Diagnostic gas analysis from oils
The following page in CHROMacademy details the benefits of headspace GC:
Question: What sample and solvent types can be used for headspace GC?
Answer: Headspace GC is amenable to the analysis of both liquids and solids.
Liquids: Viscous liquids are particularly appropriate for headspace analysis. Furthermore, liquids which contain high boiling or insoluble components, for example, blood, paint, or adhesives, are excellent candidates for headspace analysis.
Samples in water can also be excellent candidates. Although modern capillary GC columns are much more tolerant to exposure to water there is still the problem of backflash and subsequent sample carryover when using split or splitless injection modes due to the high expansion coefficient of water. This will result from the solvent vapor expanding to a volume which is larger than the liner and spilling over the top and into the inlet lines, subsequent injections will then wash these sample components back into the liner and onto the column resulting in ghost peaks.
Solids : If a solid is completely insoluble in any solvent then headspace analysis may be the best option to access the analytes of interest.
Question: If a sample is to be dissolved in a solvent, what solvents are appropriate?
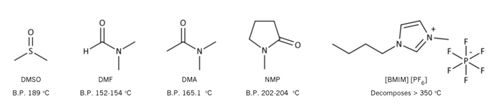
Solvents: The solvent chosen must firstly completely dissolve the sample and analytes of interest. An excellent solvent choice is water due to ease of handling and the fact that it is non-toxic. If a sample is insoluble in water an organic solvent may be required, in which case, both dimethyl sulfoxide (DMSO) and dimethylformamide (DMF) are commonly used.1 Both of these solvents are suitable as they can solubilize a large range of samples, have low volatility, and a high boiling point. If a sample is soluble in both DMF and DMSO it is preferable to use DMSO as it is less volatile, has a higher boiling point and is less toxic (Figure 1). Applications using solvents such as dimethylacetamide (DMA), N-methyl-2-pyrrolidone (NMP), ionic liquids (e.g. 1-butyl-3-methylimidazolium hexafluorophosphate, [BMIM][PF6]) have also been reported.1-3 The use of mixed diluent systems (i.e. H2O/DMSO mixtures) have also been studied and shown to affect response.4
Figure 1: Solvents suitable for GC headspace analysis.
Question:How does headspace analysis work?
The volatile analytes are evolved into the headspace by heating the sample at a fixed temperature and for a fixed length of time in a vial of known volume. Headspace analysis is an equilibrium technique and not all of the analyte will evolve into the headspace gas volume. Therefore, reproducibility of sample preparation is important with heating, agitation rate, and the ratio of the sample volume or weight to the headspace gas volume being critical for optimum, reproducible results.
The headspace gas can be sampled manually using a heated gas tight syringe (to avoid condensation) or by using an automated system employing a heated gas loop which transfers the sample to the GC column via a heated transfer line. In each case a split/splitless injector is used to facilitate gas sample introduction.
References
- K.Karthikeyan et al., Sci. Pharm. 78, 835. (2010).
- E.Hollender and E.W.Hammersley, Chromatography Today 30 (2011).
- F.H.Liu and Y.J.Jiang, Chromatogr. A 1167, 116 (2007).
- L.Kott and H.M.Chen, Pharmaceutical Technology 5, 76 (2010).
- CHROMacademy Lite Membership is FREE and it only takes two minutes to register.
- With a Lite Membership you are given access to:
- This month's webcast & tutorial
- Selected eLearning modules
- Featured CHROMacademy Content
- The CHROMacademy forum
Test drive CHROMacademy and Check out more great content available FREE to our Lite members »
Reversed Phase HPLC for the Analysis of Biomolecules
November 15th 2016Biopharmaceuticals offer great hope in treating medical conditions which are currently poorly served, at best, by traditional pharmaceuticals. It is estimated that there are over 400 biopharmaceuticals in clinical trials for in excess of 200 disease areas. The enhanced complexity and variability that comes from the size of biopharmaceuticals, allied with the intricacy of the production process, mean chromatography is employed to a much greater extent during production and release testing. The following article will introduce the fundamentals of biopharmaceutical analysis and cover the use of reversed phase HPLC in the analysis of biomolecules. A subsequent article will detail the application of HILIC, IEX, and SEC chromatography for the analysis if biomolecules.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

