Top-Down Protein Quantitation on a Triple-Quadrupole Mass Spectrometer
Top-down protein quantitation, especially using triple-quadrupole MS, but even in general, has hardly been pursued. To help fill this gap, we recently reported a systematic investigation of intact-protein quantitation using multiple reaction monitoring (MRM) on a triple-quadrupole MS system, and we believe that this approach can be a promising alternative route to consider going forward.
I was never that interested in proteomics research. I felt that it required more biochemistry and bioinformatics than analytical chemistry; also, by the time I began to consider it more seriously, some 6–8 years ago, I felt that the learning curve toward doing something new and innovative, with little prior background, was pretty darn steep. By now, the traditional bottom-up methods for protein identification and quantitation are pretty mature.
In the past several years there has been an explosion of interest in protein analysis, from a direction very different than traditional bottom-up proteomics. Most of this interest has been driven by the growing biopharmaceutical industry, but there is also increasing desire to quantitatively track protein biomarkers using technology other than immunoassays. During this same period of time, our laboratory has been working heavily on the development of new methods for trace quantitative analysis. We have been experimenting with restricted access media (1–4), in situ derivatization (1,3,5), and column screening protocols (6) to create more streamlined and more automatable methodologies for quantitation of target molecules from biological fluids. With some highly sensitive triple-quadrupole mass spectrometry (MS) instruments in our hands, we could not help but turn our head a bit toward protein quantitation.
We were extremely surprised when we performed a literature search for intact-protein quantitation using triple-quadrupole MS and did not really find anything. Such a search will yield many hits, but virtually all of the methods will eventually involve the digestion of the target protein, so that it can be quantified based on the abundance of one of its constituent proteolytic peptide fragments. Top-down protein quantitation, especially using triple-quadrupole MS, but even in general, has hardly been pursued. To help fill this gap, we recently reported a systematic investigation of intact-protein quantitation using multiple reaction monitoring (MRM) on a triple-quadrupole MS system (7), and we believe that this approach can be a promising alternative route to consider going forward.
When you think about the difficulties associated with developing unique and reproducible MRM transitions for intact proteins, like those commonly generated for small molecules, there are many considerations. The triple-quadrupole MS system is a low-resolution instrument. Thus, the precursor ion selection (one of the higher abundance multiply charged ion signals) cannot be very selective against various adducts, isotopes, proteoforms, or conformations that underlie the isolated signal window. Those precursor ions then enter a low-energy collision cell in the second quadrupole. An intact protein ion is characterized by many degrees of freedom that can readily dissipate internal energy applied through low-energy collisions with inert gases. One might not expect efficient fragmentation of the precursor ion in this environment. Finally, when product ions are selected in the third quadrupole, there is insufficient mass accuracy to assign their sequence and charge state. Even so, it can be argued that if unique and reproducible product ions are formed, then it is not really necessary to know their sequence and charge state.
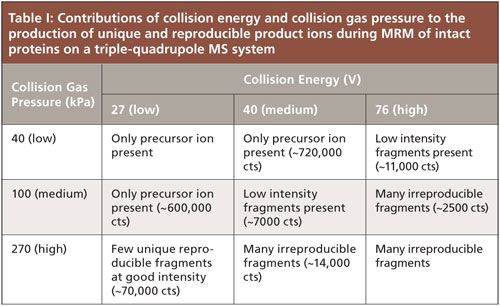
We first approached optimizing the MRM transitions much like one would do for a small molecule. The idea is to apply enough collisional energy to convert all precursor ion into product ions, for maximum sensitivity and specificity. When we did this with the intact protein ions, we observed copious irreproducible, low intensity, and low mass product ions. No unique reproducible transitions could be defined. Therefore, we performed a systematic investigation of collision gas pressure (argon) and collision energy. Table I summarizes the outcomes of these experiments. Basically, the optimal conditions occur when there is still some precursor ion remaining in the product mass spectrum. In that case, we found that fewer fragments were formed, but that these were at considerably higher abundance; they were also unique to the model proteins we tested. Performing this MRM optimization under conditions of higher collision gas pressure also proved to be essential. Thus, a framework for optimizing intact protein MRM transitions on a triple-quadrupole MS system appears to have been defined.
Strategies for optimization of sample preparation, separation, ionization, and MS detection parameters for maximal sensitivity and specificity of intact-protein quantitative determination from biological fluids has been developed to a much lesser degree than for peptides and small molecules. The detection limits we determined for proteins using MRM in a state-of-the-art triple-quadrupole MS system were not as low as one would expect for a peptide measurement; however, there can be significant advantages to avoiding protein digestion steps, as these can be incomplete and irreproducible. Absolute determinations of protein abundance are rarely possible using bottom-up methods; they are virtually always performed in a relative (healthy vs. diseased, treated vs. untreated, and so forth) manner. Proceeding in a top-down approach would allow for accurate absolute determinations of protein abundance in a sample and also direct comparisons of determinations between laboratories. Of course, some drawbacks include the inability to target specific modified forms of proteins (that is, specific proteoforms). Perhaps this disadvantage can be addressed in the future with more selective extractions and separation methods. Overall, there exists much room for fundamental research to increase selectivity and sensitivity for quantitative analysis of intact proteins by liquid chromatography–mass spectrometry. Hopefully, we have helped provide guidance on the use of an established and reliable tool, specifically MRM, to advance these efforts.
References
(1) J. Beinhauer, B. Liangqiao, H. Fan, M. Sebela, M. Kukula, J.A. Barrera, and K.A. Schug, Anal. Chim. Acta858, 74–81 (2015).
(2) B. Papouskova, H. Fan, K. Lemr, and K.A. Schug, J. Sep. Sci.37, 2192–2199 (2014).
(3) H. Fan, B. Papouskova, K. Lemr, J.G. Wigginton, and K.A. Schug, J. Sep. Sci.37, 2010–2017 (2014).
(4) S.H. Yang, H. Fan, R.J. Classon, and K.A. Schug, J. Sep. Sci.36, 2922–2938 (2013).
(5) Y. Baghdady and K.A. Schug, J. Sep. Sci.39, 102–114 (2016).
(6) D.K. Appulage, E.H. Wang, F. Carroll, and K.A. Schug, J. Sep. Sci.39, 1638–1647 (2016).
(7) E.H. Wang, P.C. Combe, and K.A. Schug, J. Am. Soc. Mass Spectrom.27, 886–896 (2016).

Kevin A. Schug is a Full Professor and Shimadzu Distinguished Professor of Analytical Chemistry in the Department of Chemistry & Biochemistry at The University of Texas (UT) at Arlington. He joined the faculty at UT Arlington in 2005 after completing a Ph.D. in Chemistry at Virginia Tech under the direction of Prof. Harold M. McNair and a post-doctoral fellowship at the University of Vienna under Prof. Wolfgang Lindner. Research in the Schug group spans fundamental and applied areas of separation science and mass spectrometry. Schug was named the LCGCEmerging Leader in Chromatography in 2009 and the 2012 American Chemical Society Division of Analytical Chemistry Young Investigator in Separation Science. He is a fellow of both the U.T. Arlington and U.T. System-Wide Academies of Distinguished Teachers.
The LCGC Blog: Historical (Analytical) Chemistry Landmarks
November 1st 2024The American Chemical Society’s National Historic Chemical Landmarks program highlights sites and people that are important to the field of chemistry. How are analytical chemistry and separation science recognized within this program?
