The LCGC Blog: Sample Preparation by Electric Field-Assisted Extraction
Extraction-based sample preparation has been recognized as a significant step in separation science. To improve the sensitivity and selectivity of the extraction, some new approaches have been introduced, including those that employ an electric field. These electric field-assisted extraction methods create another dimension for sample preparation, and are compatible with miniaturized, portable, and multifunctional analytical platforms.
Significance of Extraction in Liquid Chromatography (LC) and Gas Chromatography (GC) Applications
Analytes of environmental or biological origin occur in complex matrices, which complicates their separation and detection. For this reason, sample preparation methods such as extraction are a crucial step prior to chromatographic analysis. The goals of sample preparation technologies are purification and pre-enrichment of target analytes to be separated and then detected by the instrument. Purification reduces the concentration of interfering matrix substances, which affects separation and measurement through a decrease in sensitivity and a significant increase in maintenance, loss in chromatographic efficiency, and distortion of peak shape. In addition, high-abundance species might overwhelm the column; for example, albumin in blood may interfere in this way when evaluating lower-abundance protein biomarkers (1). Extraction accomplishes purification and pre-enrichment by partition of the analyte into or onto an extraction phase.
Advantages of Electrical Driving Force as an Auxiliary Energy in Extractions
Advances in extraction seek to improve selectivity and to boost pre-enrichment of the analyte through its increased mass transport to and uptake into or onto the extraction phase. For systems in which analytes or interferents are charged, integration of electric fields normal to the phase boundary can be leveraged to improve these features of the extraction. Electric fields can be generated and modulated by a simple pair of electrodes, and are by nature a green, cost-effective, and user-friendly addition to an extraction protocol. For this reason, the use of electric fields in extraction is an important modern trend in analytical chemistry. While many electric field–assisted separation techniques have been developed and studied in depth, such as electrodialysis, gel electrophoresis, capillary electrophoresis, and capillary electrochromatography, the use of electric fields in extraction is still an underdeveloped area.
In the context of extraction, there are three areas where an applied voltage can be employed. Electric fields can be used to drive 1) more rapid mass transport of charged analytes to the extraction phase, and 2) partition of a species with a certain charge to increase selectivity of the extraction. Further, electrosynthesis can be utilized to synthesize and prepare membranes and solid-phase sorbents for extraction. It is notable that use of an electric field to perform any of these three functions can be readily implemented in microfluidic devices to create highly integrated analytical platforms.
Fundamentals of Electric Field–Assisted Extraction
The impact of electric fields can be understood as a means to perturb equilibrium distributions of ions to favor the desired extraction. The introduction of potential gradients within each phase and at the extraction phase boundary leads to differences in electrochemical potential () that drive electromigration and partition. The electrochemical potential of species i in phase (taken here to be the sample phase) is given by its standard chemical potential
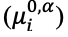
adjusted for its activity
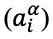
and the interaction of its charge
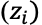
with the potential of the phase
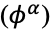
as follows:
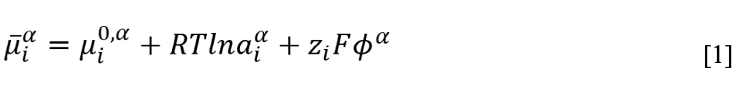
Here, R, T, and F are the universal gas constant, temperature, and Faraday’s constant, respectively. From this expression of electrochemical potential, the fundamental equations describing mass transport and the distribution of chemical species across a phase boundary, can be derived.
A spatial gradient in electrochemical potential
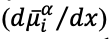
drives the flux of species i against that gradient. For example, an anionic species positioned along a gradient of increasing (positive) potential with distance
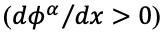
will electromigrate in the positive -direction. This outcome can be accomplished practically by application of a positive potential to the extraction phase versus the sample phase to favor electromigration of the analyte to the interface. The Nernst-Planck equation describes flux of species i by diffusion, migration, and convection. In the absence of convection or concentration gradients, flux due to migration
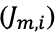
is dominant and is given in one dimension by the following expression:

This means that for a charged species, its flux due to migration is proportional to the magnitude of its charge
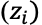
its concentration, and the electric field strength at location x.
Similarly, electrochemical potential can be used to describe the equilibrium condition for the partition of species i between two phases
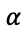
and
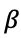
(the extraction phase)
through the equality,
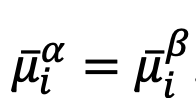
If a potential difference is applied to the extraction phase to perturb this equilibrium such that
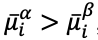
then species i will partition into the extraction phase
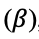
thereby increasing its activity in that phase
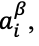
until the inequality is resolved. The partition coefficient,
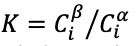
is likewise increased. For example, application of a positive potential to an extraction phase relative to the sample phase favors extraction of an anionic analyte.
The following expression describes the relative distribution of an ion between two phases as a function of potential.

Combining and rearranging these equations gives

where E is the equilibrium ion transfer potential (the Galvani potential difference between the phases), E0 is the standard potential difference of transfer that is related to the standard Gibbs energy of transfer. This equation clearly shows the relationship between the applied potential difference across a phase boundary and the ion distribution between the two phases. Note that at standard temperature, the ratio of concentrations
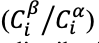
for a singly charged cation increases ten-fold for every 59 mV shift in potential.In this way, the distribution of ions can be easily manipulated by changing the applied electric potential, and thus, the extraction process can be controlled.
Promising Directions in Electric Field–Assisted Extraction
Using an electric field in extraction has several advantages, such as improved selectivity for transfer of ionized species, efficient matrix cleanup, and modulation of the sample preparation process for potentially exhaustive extraction of target species. Electric fields have been employed in both solid-phase and liquid–liquid extractions. An overview of several techniques is provided here.
Electric-field-driven liquid-phase microextraction (LPME) has been seen as a viable option for expedient supported liquid membrane (SLM) extraction of ionizable or polar species.
As shown in Figure 1(a), electrochemical liquid membrane microextraction (ELMME) is an integrated sample preparation strategy based on a three-phase system consisting of a solid support, an organic liquid, and an aqueous phase. During the process, the target analyte undergoes an electrochemical reaction that changes its redox states, making it have higher affinity for the organic phase. After extraction, the extracted species near the electrode surface can be directly determined through an electrochemical method (2). This technique has been shown to enable concentrations as low as 1 nM of bromide to be determined by measuring the reduction current of extracted bromine via cyclic voltammetry. It has excellent enrichment performance, simplicity, stability, low cost, and low consumption of organic solvents.
Interface between two immiscible electrolyte solutions (ITIES) is another electrically driven technique for sample preparation (3) and electrochemical detection (4). The electrochemical basis of ITIES is that the ions are dissolved in each electrolyte solution and then transferred across the interface at a potential corresponding to their Gibbs free energies of transfer. If the applied potential is scanned, as in cyclic voltammetry, a plot of ion current (corresponding to the transfer rate) versus potential results, and each ion yields a wave at a position and height related to its transfer thermodynamics and concentration, respectively. This method has been used for quantitative detection of a series of model redox-inactive tetraalkylammonium cations, and for identification of the transient adsorption of these cations at the interface during the ion transfer process. One of the clear benefits of ITIES is efficient extraction in a short time and at very low voltages, which is significant for the analytes prone to electrochemical degradation. Recently, this method has been utilized for quantitative and qualitative detection of neurotransmitters such as acetylcholine. While ITIES has not been integrated with chromatographic methods, its ability to simultaneously manipulate the extraction of, identify, and quantify analytes would make it a tunable and information-rich sample preparation approach.
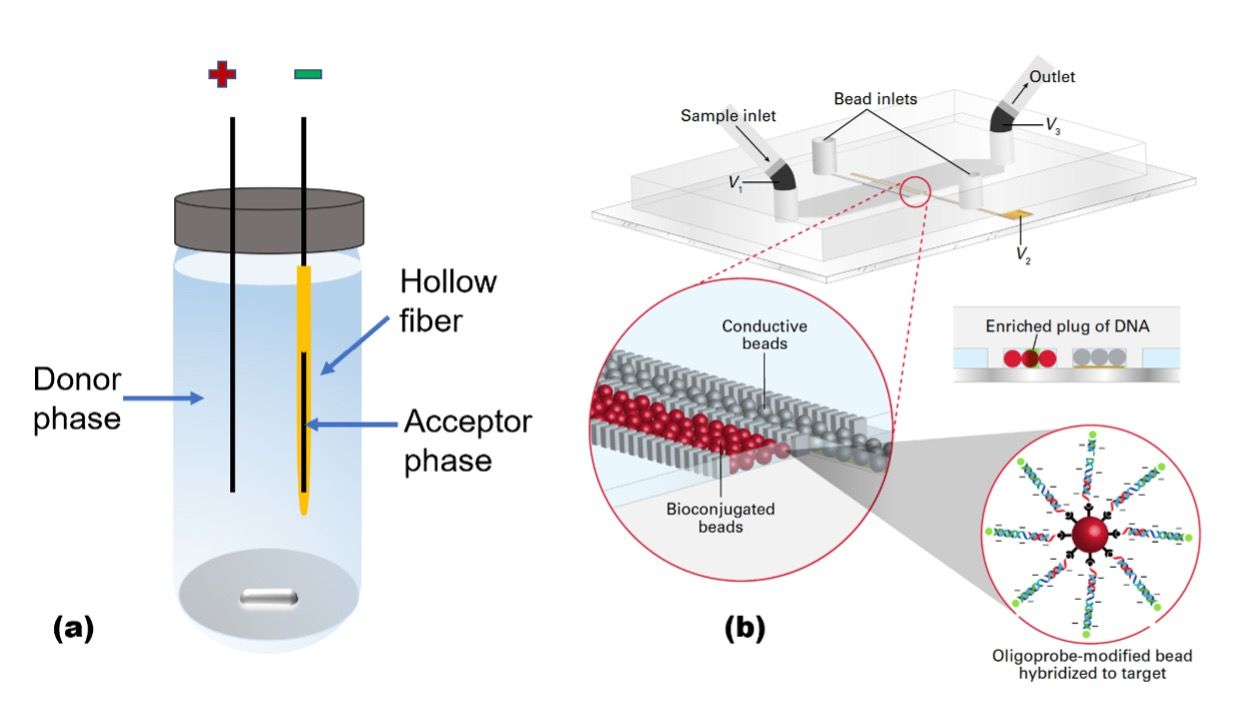
Figure 1: Two examples of electric field–assisted extraction: (a) electrochemical liquid membrane microextraction (ELMME), which is based on a three-phase system consisting of a solid support, an organic liquid, and an aqueous phase; and (b) bead-driven ion concentration polarization (ICP)–driven extraction.
Several promising applications of electrically enhanced solid-phase extraction techniques have been developed. These include electrochemical synthesis of solid-phase sorbents and electric field-driven enhancement of extraction efficiency. In sorbent synthesis, electrochemical methods enable tailoring of several key factors including thickness, conductivity, oxidation state, and morphology of a sorbent coating. Recently, a new trend of using heterocyclic compound functionalized ionic liquid polymer films has developed (5,6), and the thickness of these polymer films are tunable by using chronoamperometry.
Electric fields have been employed to enhance solid-based extraction by either direct manipulation of extraction or by migration of charged species to the interface during the extraction phase or away from the interface during the elution stage. Compared to traditional solid-phase extraction, electric field-enhanced extraction provides a new dimension to separation art, making it more versatile and dynamic.
Techniques that employ non-linear (graded) electric fields for electrokinetic extraction are less developed and have the potential to access new classes of analytes and to more dramatically enhance extraction efficiency. These techniques include dielectrophoresis (DEP), for manipulation of polarizable particles (including those lacking a net charge), and electric field gradient focusing (EFGF) methods. Most prevalently, these electrokinetic methods have been integrated with biological analysis. For example, DEP has been used to selectively accumulate proteins, organelles, viral capsids, and biological cells, among others.
An electric field gradient can be formed by many approaches including direct manipulation of potential through electrode arrays, tapered channel geometries, or by spatial variation in electrolyte concentration. Ion concentration polarization (ICP) accomplishes enrichment and depletion of electrolyte ions at opposite ends of an ion permselective junction connecting two microchannels. A voltage bias applied across the ion depletion zone (IDZ) will yield, at the IDZ boundary, a steep electric field gradient that can be leveraged for counter-flow focusing of ionic analytes. Generally, ICP-enhanced microextraction is accomplished by accumulation of target analytes into focused plug and extraction of the focused plug into a separate phase (7). For example, ICP-based focusing followed by encapsulation of the analyte into droplets was reported to yield 103-fold enrichment (8). Recently, by using an out-of-plane 3D electrode to drive faradaic ICP as shown in Figure 1(b), two limitations of traditional ICP—a loss of efficiency from leaking of the analyte around the IDZ and unwanted mixing from electroconvection—have been addressed (9). Notably, in this method, the analyte is focused within a packed bed of microbeads, which provides a solid support for extraction.
Conclusion
We have briefly summarized applications of electric fields as a means to modulate extraction. These methods enrich target analytes, improve sensitivity, and hold the potential to integrate separation and detection. These methods are broadly applicable to biological, environmental, and pharmaceutical samples. These advantages warrant further investigation into scaling and commercialization of these methods and development of new non-linear electrokinetic techniques for enrichment of polarizable particles and charged analyte prior to extraction.
References
1. D.R. Haudenschild, A. Eldridge, P.J. Lein, B.A. Chromy, Biochem Bioph Res Co 455 (1–2), 84–89 (2014).
2. J. Hao, Y. Zhu, G. Li, X. Jia, Q. Qu, Microchim Acta 167 (3-4), 267–272 (2009).
3. M. F. Suárez-Herrera, M.D. Scanlon,Anal Che 92 (15), 10521–10530 (2020).
4. H.D.J etmore, C.B. Milton, E.S. Anupriya, R. Chen, K. Xu, M. Shen, Anal Chem 93 (49), 16535–16542 (2021).
5. A.M. Devasurendra, C. Zhang, J.A. Young, L.M.V. Tillekeratne, J.L. Anderson, J.R. Kirchhoff, ACS Appl Mater Inter 9 (29), 24955-24963 (2017).
6. H. Chen, J.L. Anderson, R.K. Anand, R. K., ACS Appl Mater Inter (2022).
7. A. Krishnamurthy, R.K. Anand, Trac-Trend Anal Chem 148, 116537 (2022).
8. V.A. Papadimitriou, S.A. Kruit, L.I. Segerink, J.C.T. Eijkel, Lab Chip 20(12), 2209–2217 (2020).
9. B. Berzina, S. Kim, U. Peramune, K. Saurabh, B. Ganapathysubramanian, R.K. Anand, Lab Chip (2022).
Han Chen

Han Chen received her BS degree in Beijing Normal University (Beijing) in 2018. She currently attends the Iowa State University as a PhD student co-advised by Dr. Jared L. Anderson and Dr. Robbyn K. Anand. Her research involves incorporating the functionalized polymeric ionic liquids into microfluidic platform as a means to facilitate single cell analysis.
Robbyn K. Anand

Robbyn K. Anand is the Suresh Faculty Fellow and Carlyle G. Caldwell Endowed Chair in Chemistry at Iowa State University where she joined the Department of Chemistry as an Assistant Professor in August 2015. She earned her PhD in 2010 from the University of Texas at Austin under the guidance of Prof. Richard M. Crooks with the support of an NSF Graduate Research Fellowship. She developed microfluidic devices employing bipolar electrodes for electrokinetic focusing of charged species. Then, as an NIH Postdoctoral Fellow, she worked with Prof. Daniel T. Chiu at the University of Washington on the capture and analysis of circulating tumor cells. At Iowa State, Prof. Anand has led the development of a technology for the selective isolation and analysis of single cells with the aim of obtaining information that can improve outcomes in cancer therapy. Her research group has also advanced methodologies for separations in water-in-oil droplets and in complex media (for example, blood plasma). During this time, Prof. Anand founded the Midwest Women Chemists Retreat, an annual event aimed at the retention of women in the chemical enterprise.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




