SPE Capabilities Tested with Sponge-Nested Polymer Monoliths
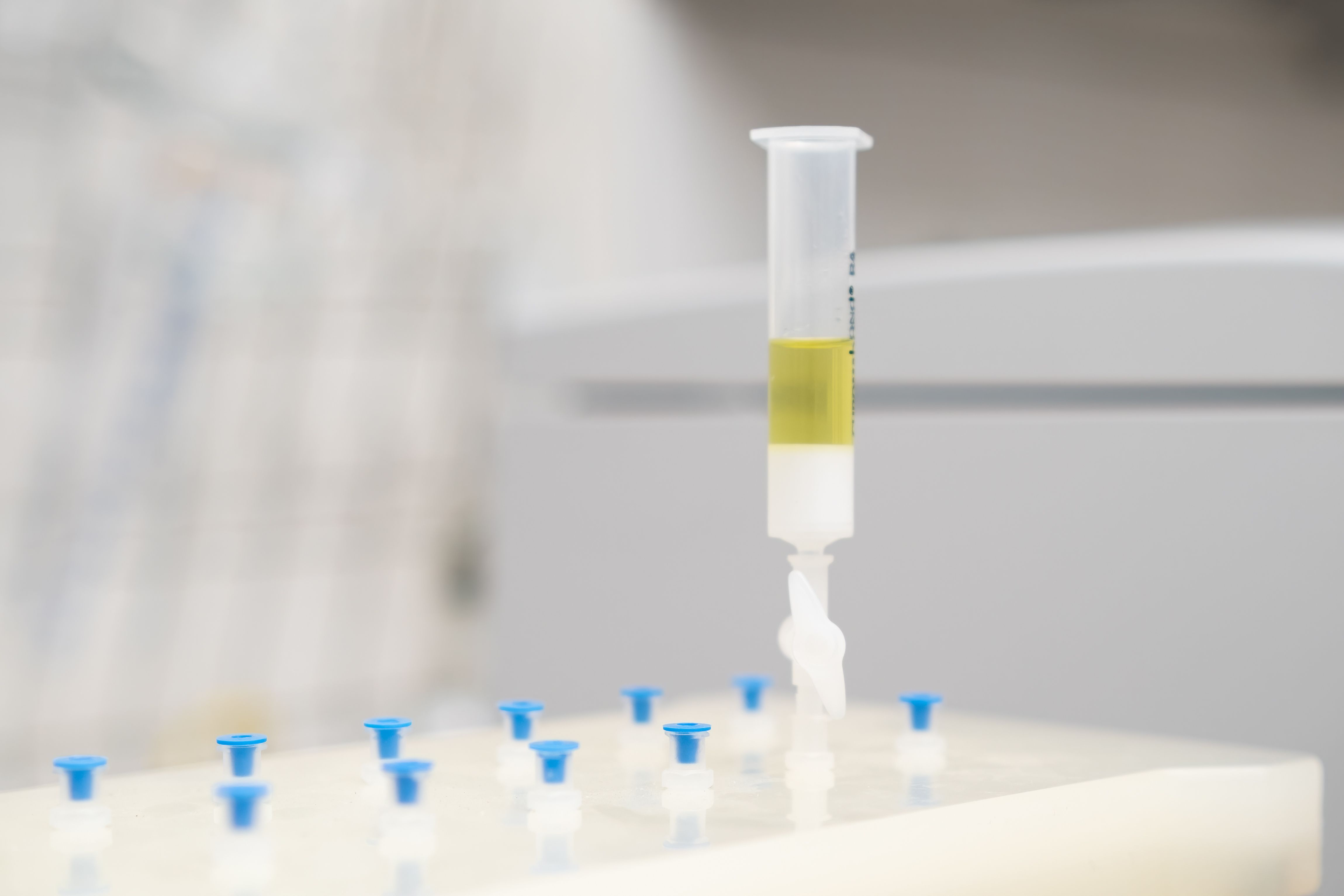
Divinylbenzene (DVB) monoliths were nested within a melamine-formaldehyde sponge for solid-phase extraction (SPE) of bisphenols from aqueous solutions.
A new study in the Journal of Separation Science has evaluated the performance of a custom method of solid-phase extraction (SPE) of bisphenols from an aqueous medium (1). The method makes use of increasingly popular polymer monoliths, which the authors of the study said aid sample preparation because of their high porosity, pH stability, and ease of use. Using divinylbenzene (DVB) as a single cross-linker, the monoliths in this experiment were nested in a melamine-formaldehyde sponge, the combination of those two compounds in foam form having previously been reported as an effective support.
Vacuum Solid Phase extraction SPE manifold with catridge for sample preparation in chemical laboratory. | Image Credit: © vladim_ka - stock.adobe.com
SPE is a sample preparation technique widely used in analytical chemistry to isolate and concentrate target compounds from complex mixtures. In SPE, a stationary phase, typically a solid sorbent material, is packed into a column. The test sample is then introduced onto the column, and undesired components in the sample are washed away using a solvent. The target compounds are retained on the sorbent, and subsequently eluted, providing a purified and concentrated sample suitable for further analysis.
According to authors Natalia Morales, Stuart C. Thickett, and Fernando Maya, all from the Australian Centre for Research on Separation Science (ACROSS) at the University of Tasmania in Hobart, Tasmania, Australia, polymer monoliths have attracted attention and widely accepted usage in SPE over the last quarter-century (1). These singular pieces of porous material that fill the entire volume of a mold provide versatile variety in shape, size, and preparation by organic, inorganic, or hybrid-composition compounds.
Sponge-nesting was first developed for silica monoliths, with the monolith prepared inside a melamine-formaldehyde sponge; this resulted in a highly customizable yet robust and sizable monolith with no resultant structural damage (1). These researchers tested the process instead with polymers, noting that large polymer monoliths can be damaged when adapted to a different shape or vessel. In this case, DVB was used to achieve internally cross-linked networks that incorporated micro- and mesopores on the globules of the monolith, increasing surface area to more than 400 m2/g. However, the researchers tested the tunability of the sponge-nested monolith’s size by cutting it (0.125 cm3) into four smaller, identical-sized monoliths (0.03125 cm3 each).
The sponge-nested monoliths were then used for the extraction—in three different modes, vortex mixing, magnetic stirring, and orbital shaking—of bisphenols, which are commonly involved in the fabrication of hard plastics including polycarbonates (1). These compounds can be found in chemicals both industrially and in the household, leading to exposure in the environment and drinking water, according to this study (the bisphenols extracted here were, in fact, taken from water samples). The researchers indicated that this is troubling because bisphenols are considered endocrine disruptors and can alter the functions of that system, causing various side and health effects in humans and other organisms.
The results of the study showed that out of the three performed extraction modes, vortex mixing yielded what the researchers called a “comparable” recovery of bisphenols, between 39% and 81%, in a quarter of the usual time—30 min versus 2 h (1). The division of the monolithic cube into four smaller parts, meanwhile, resulted in increased contact area with the sample and therefore a reported 16% to 21% increase in extraction efficiency. Buoyed by what the study found, the researchers suggested that further experimentation should be done with the intent of improving extraction selectivity and sensitivity, all while continuing to cut down on runtime and increase efficiency.
Reference
(1) Morales, N.; Thickett, S. C.; Maya, F. Sponge-Nested Polymer Monoliths: Versatile Materials for the Solid-Phase Extraction of Bisphenols. J. Sep. Sci. 2023, 2300378. DOI: 10.1002/jssc.202300378
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)