Rapid Assessment of Biosimilarity of Therapeutic Proteins Using ZipChip
Sufficient evidence of analytical similarity between a biosimilar candidate and the reference biologic can significantly reduce the burden of clinical approval of a biosimilar. In this study, a method for rapidly assessing the structural similarity of a biosimilar with the reference biologic (innovator) using microfluidic CE-ESI-MS is presented. The separation method is requires no optimization and can be used for rapidly assessing charge heterogeneity of biologics under native conditions.
Monoclonal antibodies (mAbs) are an important class of biologics for the treatment of critical diseases. Assessment of the heterogeneity of mAbs is essential for product quality control, especially for biosimilars. Before in-depth characterization, a quick study for fast assessment of charge heterogeneity comparison between the innovator and biosimilar can help save time and reduce bottlenecks with mass spectrometer usage for busy analytical laboratories.
Capillary zone electrophoresis (CZE) coupled with mass spectrometry (MS) is well suited for the assessment of mAb heterogeneity with the ability to assess important product quality attributes, such as molecular weight, N-glycan profiling, and C-terminal lysine variant differences between samples. While capable of in-depth mAb characterization (1), CZE–MS can also be used to quickly assess the quality and authenticity of a mAb (2).
In this study, cetuximab samples were analyzed to compare structural similarity and heterogeneity (an innovator cetuximab, and a biosimilar). Rapid assessment was accomplished with minimum method development for both the CE separation and MS detection methods.
Materials and methods
Sample Prep
Innovator cetuximab and biosimilar were buffer exchanged into Native Antibodies BGE assay kit and were further diluted to 2 mg/mL and 1 mg/mL respectively using the BGE.
Instruments
A ZipChip® (908 Devices Inc.) was used as the microfluidic CZE inlet. The mass spectrometer used was the Orbitrap Exploris 240 MS with BioPharma Option (Thermo Fisher Scientific).
ZipChip Method
ZipChip Protocol: Intact Charge Variant Analysis (3)
Assay Kit: Native Antibodies BGE
Chip type: ZipChip HRN
Injection volume: 1 nL
Analysis time: 20 min
Figure 1: ZipChip mounted to a Thermo Scientific Orbitrap mass spectrometer.
MS Method
Scan Range (m/z): 2500–8000
Resolution setting: 30,000 at m/z 200
Sheath gas: 2
In source-CID (V): 125
Normalized AGC target (%): 300
RF lens (%): 60
Microscans: 5
Results and Discussion
Cetuximab is a highly complex mAb due to the presence of four glycosylation sites—two in the Fc region and two in the Fab region. The Fab glycans on cetuximab are larger, more complex, and sialylated. The presence of several sialic acid groups on the N-glycans, together with other modifications of the primary sequence, are responsible for a very complex charge variant profile of cetuximab.
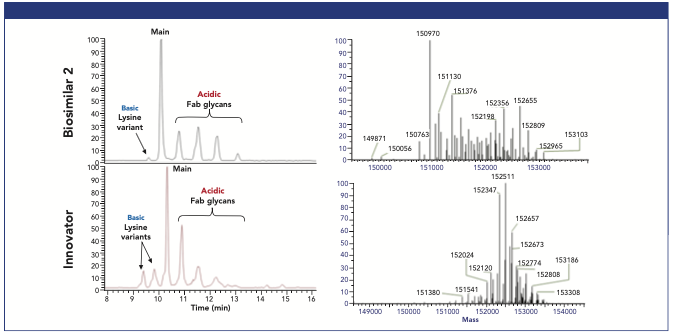
Figure 2 shows a comparison of charge variant profiles and deconvoluted mass spectra for biosimilar and innovator cetuximab. Based on the charge variant profile alone, the biosimilar appeared to be similar to the innovator—in that the molecule showed both acidic and basic species in the base peak electropherogram. Six baseline-resolved variants were observed in the biosimilar as opposed to eight variants in the innovator. There was a single basic variant with a relative abundance (<5% in comparison with the main variant) that was much lower than the relative abundance of the basic variants (~15–20%) for the innovator cetuximab. The profile also showed four acidic variants with varying abundances. The deconvoluted mass spectral data revealed that there were differences between the two samples. For example, the molecular weight of the main variant was less than that of the innovator by 1541 Da, indicating that they were different species. Biosimilar 2 shared only 30% of all the detected and deconvoluted masses with the innovator. These differences indicated that the two molecules had different glycosylation profiles and possibly different amino acid sequences as well.
Figure 2: Comparison of charge variant profiles of cetuximab biosimilar (top left) vs cetuximab innovator (bottom left) and their respective deconvoluted mass spectra, cetuximab biosimilar (top right), cetuximab innovator (bottom right).
Conclusion
A CZE–ESI–MS-based rapid charge variant profiling of a cetuximab innovator and its biosimilar under native conditions was performed with ZipChip-Orbitrap Exploris 240 MS. Quick assessment and comparison of different mAb samples was easily achieved without optimization of separation. This demonstrates that the ZipChip-MS is an easy and efficient technique for quick analysis of biologics for quality control and assessing biosimilarity.
References
(1) F. Fussl, A. Trappe, S. Carillo, C. Jakes, and J. Bones, J. Anal. Chem. 92(7), 5431–5438 (2020).
(2) 908 Devices, ZipChip Application Note 8.5 “A ZipChip Based CZE–MS Analysis for Quick Assessment of Biotherapeutics.”
(3) 908 Devices, https://908devices.zendesk.com, (2021).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.
Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)
