Online UPLC Method for the Support of Cleaning Validation and the Routine Monitoring of Cleaning Procedures
During the manufacture of active pharmaceutical ingredients (APIs), the formulation of drug substances, and therapeutic fill and finish, the removal of residues from manufacturing equipment is performed by a series of cleaning procedures. It is imperative that the production equipment be properly cleaned in order to avoid cross-contamination of drug products.1-3 The efficiency of the cleaning procedures must be demonstrated through cleaning validation. This involves demonstrating that residual API, starting material, intermediates, and impurities have been removed from the production equipment.
Tanya Jenkins, Waters Corporation, Milford, MA, U.S.
INTRODUCTION
During the manufacture of active pharmaceutical ingredients (APIs), the formulation of drug substances, and therapeutic fill and finish, the removal of residues from manufacturing equipment is performed by a series of cleaning procedures. It is imperative that the production equipment be properly cleaned in order to avoid cross-contamination of drug products.1-3 The efficiency of the cleaning procedures must be demonstrated through cleaning validation. This involves demonstrating that residual API, starting material, intermediates, and impurities have been removed from the production equipment.
During the cleaning procedure development and validation process, it is important to evaluate the effectiveness of each cleaning step in the overall process to adequately understand at what point the equipment becomes clean. It is also important to confirm that an unclean piece of equipment yields an unacceptable result.
Once the cleaning method has been validated, routine equipment cleaning should be monitored. Typically samples (either swabs or wash solvents) are taken to an off-line quality control (QC) laboratory for analysis. The time it takes to receive results from the off-line laboratory can range from hours to days. During this time, the production equipment must sit idle. If laboratory results are positive for API residues, the cleaning process and subsequent off-line QC testing must be repeated, increasing the amount of time the manufacturing equipment sits idle.
An analytical method is required that can simultaneously monitor all of the components present on the production equipment at the required safety levels. The acceptance criteria for API residues vary according to the potency of a drug substance. In general, most processes aim to have a low safety limit in the 10 ppb to 1 ppm range (10 ng/mL to 1 μg/mL). In order to achieve these limits, sensitive analytical techniques are required.4
This application note describes a fast, online, UltraPerformance Liquid Chromatography (UPLC®) method that monitors wash solvents directly from a sampling point on the manufacturing equipment. By monitoring wash solvents online, the point at which the API has been removed from the production equipment can be determined. This can reduce the volume of wash solvent required, particularly on equipment that is used for multiple APIs and where a cleaning procedure was developed against the "worst case." By gaining a better understanding of the cleaning procedure and reducing the dependency on off-line QC results, the time that the equipment must be taken off-line for cleaning and verification can be substantially reduced.
The results from the online method are compared to those obtained by testing swabs and wash solvents at an off-line UPLC system. The PATROL™ UPLC Process Analyzer Online System (Figure 1), which includes specialized, integrated hardware and software, was designed to be utilized in a manufacturing environment and provides near real-time analysis of inprocess samples, both online and atline.

Figure 1. The PATROL UPLC Process Analyzer System.
EXPERIMENTAL
Reaction conditions
Cleaning was performed on reaction vessels used for the conversion of acetylsalicylic acid (ASA) to salicylic acid.5 A solution of 0.3 g/L ASA in water was prepared in a 1-L reaction vessel. Nitric acid (10 mL) was added to the reactor, which was placed in a heated bath at 75 °C. After 2 hours the temperature was reduced to 7 °C, and after 2 additional hours the reactor was removed from the bath. The reactor was then emptied in preparation of cleaning.
Cleaning procedure
The final cleaning procedure included three wash steps using 100 mL of 50:50 water/methanol to clean the inside of the reactor, and two wash steps to clean the exit port of the reactor using 200 mL of the same solvent. Wash solvents after each step were sampled and analyzed to monitor the cleaning progress. Swabs were used to assess the reactor cleanliness throughout the procedure and also after the final cleaning step to ensure levels were below acceptable limits.
Quantitative methodology
Calibration curves for the starting material and final product were based upon four standards at levels ranging from 10 ng/mL to 50 μg/mL, depending on which step in the cleaning process was being assessed. The linear range was determined by analyzing 12 standards across the entire concentration range. The limit of detection (LOD) was defined as s/n=3 and the limit of quantification (LOQ) was defined as s/n=10.
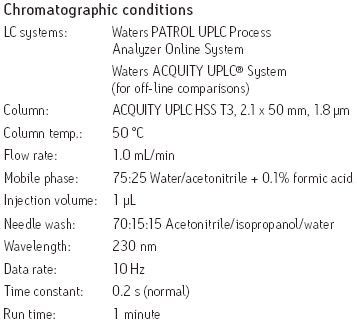
RESULTS AND DISCUSSION
Chromatographic method
A fast isocratic method was developed for online monitoring of the wash solvents. The final method had a 60-second run time with an inject-to-inject cycle time of 160 seconds, resulting in near real-time analysis. The method provided excellent resolution of the starting material, final product, and the two critical process impurities. An example of the chromatography for a standard and the first reactor wash step are shown in Figure 2.

Figure 2. Example chromatograms for a standard (A); and the first wash step (B) containing starting material, final product, and two process impurities.
Limits of detection/quantification and linear range
To ensure that the method met sensitivity requirements and that the linear range was sufficient to quantify across the required range, a calibration curve was generated from 10 ng/mL to 50 μg/mL. The calibration curve used a 1/x weighting to ensure good quantification at low concentration levels. Exceptional linearity was observed with R2 values in excess of 0.999 for the curve, which extended across more than three orders of magnitude (Figure 3). The final method had excellent limits of detection, as low as 24 ng/mL (Table 1). LOD and LOQ were determined by plotting amount versus s/n for the low level standards. For each analysis, only 1 μL was injected on column, indicating the method was sensitive enough to detect levels as low as 24 pg on column.

Figure 3. Calibration curves for the starting material and final product (10 ng/mL to 50 μg/mL).
Assessing online monitoring by UPLC
To demonstrate the viability of using the PATROL UPLC Process Analyzer Online System for the support of cleaning validation and the routine monitoring of cleaning procedures, equivalency to off-line results must be determined.

Figure 4. Standard injection near LOQ.
A cleaning protocol for the reactor was developed and residual levels were assessed after each step by both online and off-line analysis. The final cleaning procedure consisted of three wash steps inside the reactor (protocol A) and two wash steps at the outlet (protocol B). The residual levels determined by tests at each step are listed in Table 2. It is important to note that if the final product was detected by off-line analysis (wash solvents or swabs), it was also detected by online monitoring.

Table 1. LOD and LOQ of the reaction components.
Additionally, if the online results indicated the equipment was clean, the subsequent off-line analyses (wash solvents and swabs) also indicated cleanliness. The PATROL UPLC System was an extremely useful tool in developing the cleaning protocol, as the level of contamination could quickly and easily be determined at each cleaning step.
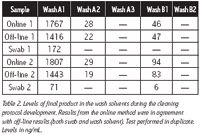
Table 2. Levels of final product in the wash solvents during the cleaning protocol development. Results from the online method were in agreement with off-line results (both swab and wash solvent). Test performed in duplicate. Levels in ng/mL.
Once the final cleaning procedure was developed, the repeatability of the PATROL UPLC System to routinely monitor the cleaning process was assessed. The reactor was cleaned four times, and the results of online and off-line monitoring were consistent for determining the presence of both the starting material and final product (Table 3). The final results indicate that if residue was not detected in the A wash steps, the inside of the reactor was clean; and if residue was not detected in the B wash steps, the outlet of the reactor was clean (as confirmed by swab analysis).

Table 3. Levels of starting material and final product (ng/mL) as determined online by the PATROL UPLC System and an off-line method. All corresponding swabs after the final wash step were also clear.
Benefits of online monitoring by UPLC
Routine online monitoring of the cleaning procedures for manufacturing equipment is more effective than traditional off-line tests. A reactor used for multiple APIs can be cleaned in-place and analyzed to ensure it meets specifications rather than over-washing to "worst-case," which utilizes excess solvent and time. It also eliminates the risk of equipment failing repetitive cycles of off-line QC testing and sitting idle while the cleaning procedures are repeated.

CONCLUSION
- The results obtained by online monitoring with the PATROL UPLC System were consistent with those determined by off-line analysis.
- The PATROL UPLC System was able to monitor low ng/mL levels required to support cleaning validation.
- The large linear dynamic range of the PATROL UPLC System provides the means to monitor reactions at high concentrations and monitor the low levels required for cleaning procedures on the same instrument.
- The PATROL UPLC Process Analyzer Online System provides a highly effective solution to support cleaning validation and the routine monitoring of wash solvents from the cleaning of manufacturing instrumentation.
- Use of the PATROL UPLC System for online monitoring reduces manufacturing equipment down-time for cleaning procedures.
References
1. Guidance for Industry: Manufacturing, Processing, or Holding Active Pharmaceutical Ingredients, FDA Draft. March 1998.
2. Cleaning Validation in Active Pharmaceutical Ingredient Manufacturing Plants, APIC. September 1999.
3. Guidance on Aspects of Cleaning Validation in Active Pharmaceutical Ingredient Plants, APIC. December 2000.
4. Fountain KJ, van Wingerden M, Diehl DM. A High Throughput UPLC/MS Method in Support of Cleaning Validation Studies. Waters. June 2007; 720002171en.
5. Jenkins T. Online Reaction Monitoring of Inprocess Manufacturing Samples by UPLC. Waters. May 2008; 720002605en.
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.





