The LCGC Blog: Using Hydrogen Carrier Gas with Mass Spectrometric Detection
Our recent discussion on the use of hydrogen as a carrier for gas chromatography applications elicited many questions and comments, however one common question was “what are the considerations for using hydrogen carrier with MS detectors?”
Our recent discussion on the use of hydrogen as a carrier for gas chromatography applications elicited many questions and comments, however one common question was “What are the considerations for using hydrogen carrier with MS detectors?”
The answer is: “It’s possible but not straightforward!” In my experience, it would be fair to say that I’ve seen a spectrum of issues when switching to a hydrogen carrier but rarely have any of the issues been insurmountable if one is determined to make the change. While the drivers for change may not be convincing presently, as the cost of helium increases and availability becomes more limited, we will need to get serious about making the change.
What follows is a brief discussion on the known issues and various approaches to adopting hydrogen as carrier for GC–MS applications, which I hope helps to inform your decision and practice. Practical information from end-users is scant, so it would be great to hear from readers on their own experiences to help inform the debate.
Flow rates and column dimensions
Hydrogen is around half as viscous as helium, which, when coupled with the fact that there is a vacuum at the column outlet, can mean that the head pressures required to generate the desired column flow rate can be too low for instrument pneumatic controls to deliver in a reproducible fashion. In most cases the answer is to use a shorter, narrower internal diameter column, to achieve good linear velocity (>35 cm sec-1) at lower volumetric flow rates and head pressures that the instrument pneumatic systems can reproducibly deliver. Flow rates of between 0.3 and 0.4 mL/min are prevalent in literature, with linear velocity in the 40 cm sec-1 range or higher, which is appropriate as hydrogen is around twice as efficient as helium in this range.
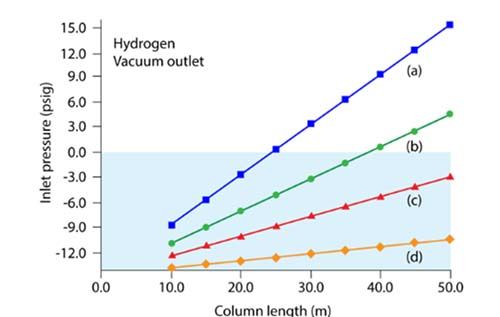
Figure 1: Plots of inlet pressure vs. column length, with hydrogen carrier gas, and vacuum compensation on. Column inner diameters (mm): (a) 0.20, (b) 0.25, (c) 0.32, (d) 0.53; column temperature: 50 °C; average linear velocity: 40 cm/s. The blue shaded area designates negative inlet pressures.
Delivering lower volumes of hydrogen into the ion source offers other advantages as we will see later.
Two further considerations result from the use of narrow internal diameter columns:
- The stationary phase capacity of the column will be more limited and therefore injection volumes or sample concentration may need to be reduced
- Translation software may be required in order to maintain retention times between the original and new methods. As linear velocity changes the rate of change of temperature per unit time may need to be adjusted in order to maintain the elution order of the analytes. At the very least, you will need to verify the identity and elution order of analytes under the new column conditions.
A further impact of the use of lower head pressures is the rate at which the sample is transferred to the GC column, which can be further exacerbated using a lower-viscosity carrier gas. In order to avoid issues of backflash (overfilling of the inlet liner due to solvent expansion, leading to carry-over and/or irreproducible peak areas), a pressure pulsed injection may be considered, which applies an increased head pressure to the system during the injection phase of analysis.
MS Vacuum System
Due to the lower viscosity of hydrogen, MS vacuum pumps must work harder in order to maintain the required vacuum levels. Some vacuum pumps are not capable of achieving the required vacuum.
Insufficient vacuum within the detector will result in lower inherent instrument sensitivity and may also risk a build-up of hydrogen within the detector, which is an obvious safety concern but will also increase the possibility of reactions between analyte ions and hydrogen carrier (more on this subsequently). One should monitor vacuum level and ensure the minimum vacuum level is being achieved (one manufacturer recommends a vacuum no lower that 5 x 10-5 torr for example).
More recently, instruments have been designed with pumping systems capable of achieving the required vacuum levels with hydrogen carrier, however one should check with the manufacturer prior to making the change to hydrogen. It is also important that the roughing pump outlet is directed into a vented fume-hood or outside.
MS Source Components
Most manufacturers will either offer a hydrogen specific ion source design or will provide change-out components to ensure that the source is optimally configured. In most cases, this will be componentry which ensures the optimum ionization efficiency within the ion source combined with “slotted” components to allow the most effective pumping-away of the carrier gas from within the ion source.
There are also ad. hoc. reports of hydrogen carrier altering the metallurgic properties of certain metals used within ion sources, as well as changes to the way in which magnets within the ion source, operate. Once again, some of these components may need to be changed in order to work with hydrogen carrier. Contact your manufacturer for more advice on this subject.
Conditioning
Hydrogen is more likely than helium to displace contaminants that build up on roughened or unswept surfaces within the GC system and the mass spectrometer. For this reason, spectra acquired soon after changing the carrier gas will appear very noisy.
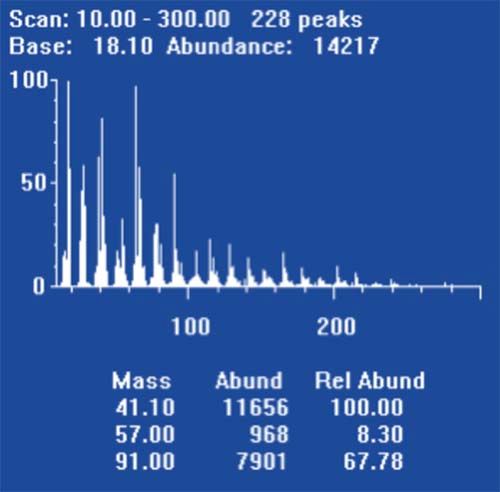
Figure 2: “Noisy” spectrum obtained from PFTBA tune compound “scan” on switching to hydrogen carrier gas.
The manufacturer will have a suggested “conditioning” routine which might include running at the highest source temperatures, reduced detector (electron multiplier) voltage with the filament turned on, overnight. While in the early days of experimenting with hydrogen carrier for MS detection, we experienced conditioning periods of up to one week to be necessary, most manufacturers now suggest overnight conditioning routines.
Further, if the system is not properly conditioned, very pronounced chromatographic peak tailing may occur. In some cases, this tail may be chromatographic in origin (such as secondary interaction of the analyte with active sites within the system) but occasionally this may be due to the formation of analyte degradation products due to ion-molecule reactions with hydrogen in the ion source. In our experience this phenomenon will reduce significantly after proper conditioning.
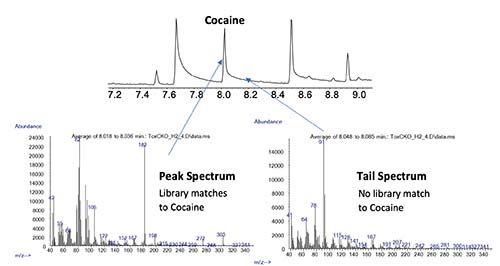
Figure 3: Spectra from a tailing GC–MS peak that reveals that the tail is contains a separate species, postulated to be a degradation product. (Figure courtesy of Agilent Technologies, Santa Clara, USA)
In our experience, the use of hydrogen does require more conditioning pre-analysis, even on standing overnight, but this does not significantly impact on the “practical usability” of hydrogen as a carrier.
Sensitivity
Typically, a factor of between 2–5 fold reduction in sensitivity is to be expected when using hydrogen as the carrier gas. While baselines may be noisier, the improvements in peak efficiency due to the higher inherent efficiency of the hydrogen carrier and the (typically) shorter retention times, somewhat offset this.
The reduction in sensitivity is typically attributed to the higher source pressures (lower vacuum) associated with the decreased pumping efficiency when using hydrogen. Therefore the use of optimum volumetric flow into the ion source, hydrogen-approved ion source components, and highly efficient pumping systems is a pre-requisite, especially when carrying out trace analysis.
Ion-molecule reactions
It is a fact that hydrogen is inherently more reactive than helium, and it is possible, especially at elevated source pressures, to induce chemical ionization (CI) “like” reactions between analytes and hydrogen ions within the source to form an M+1 ion. Further, there are some reports which cite hydrogen-induced degradation or in-source reactions such as the reduction of nitrobenzene to aniline or hydrogenation of unsaturated species, however a literature search of ion-molecule reactions in electron ionization (EI) GC-MS using hydrogen carrier does not reveal a significant amount of supporting information. Further, in our own work we have seen only one or two real examples of changes in analyte composition which can be attributed to hydrogen ion-molecule reactions, however the general advice would be to assess the possibility of ion-molecule reactions when translating established method or developing new ones.
It is possible when using chlorinated solvents such a dichlorobenzene or carbon disulphide to encounter formation of hydrochloric acid at the elevated temperatures within the GC inlet, and presumably the MS ion-source if the temperature is high enough. This acid has the potential to strip deactivation away from the liner and GC column, leading to tailing peaks or problems with quantitative reproducibility or linearity. For this reason, working with these solvents when using hydrogen as the carrier is to be avoided. One should also consider using a highly deactivated liner with bottom restriction, to avoid contact of the analyte and solvent with the hot metal surfaces at the bottom of the inlet and to change GC consumables regularly.
MS Spectra and Tuning
While most MS spectra remain very similar, there is a tendency for the quality of spectral “match” to drop when using hydrogen carrier gas. Typically, this doesn’t affect positive identification, and we have only rarely experienced a situation where qualifier ion ratios need to be adjusted when quantifying in Selected Ion mode, however these limitations should be acknowledged and considered when converting.
Further, while not all spectra are affected in the same way, there are examples where appreciable changes occur in certain ion ratios between spectra obtained in either helium or hydrogen carrier gas. There are also examples where extra peaks are seen (usually at low abundance) in the spectra acquired with hydrogen as the carrier.
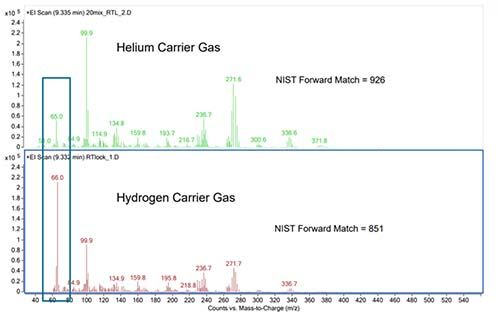
Figure 4: Spectral differences for EI-GCMS analysis of Lindane. (Figure courtesy of Agilent Technologies, Santa Clara, USA)
In terms of tuning, in our experience, electronic ionization tuning characteristics obtained using perfluorotributylamine (PFTBA, HEPTACOSA) are usually consistent with those obtained using helium carrier, and in most instances a satisfactory tune can be obtained, for example, the tune “passes” and is within tolerance of the instrument manufacturers tune targets.
However, there are regulatory methods that use alternative tune compounds to PFTBA and also require certain relative ion abundances to be within defined tolerances. Decafluorotriphenylphosphine (DFTPP) for EPA method 8270 appears to give reasonable tune agreement between the two carrier gases, however, it is reported that bromofluorobenzene (BFB), used for EPA methods 524, 624, and 8260B for example, can suffer from skewing of some key ion ratios when using hydrogen carrier, especially in qualifying the relationship between masses 95 and 96 m/z.
In summary, as I pointed out earlier, possible but not straightforward, major considerations for converting to hydrogen carrier gas when using MS detectors, are:
- Use of reduced dimension columns at higher linear velocity but lower volumetric flow
- Use of efficient vacuum pumps, rated for use with hydrogen and optimize vacuum to the best levels possible
- Confirm if a source change or source component change is required
- Ensure a proper conditioning routine is undertaken
- Use method translation software to ensure peak retention order is maintained and check peak identities to confirm
- Be alert to the possibility of ion molecule reactions and investigate or mitigate where necessary.
- Confirm spectral integrity and consider impact on quantitative ion ratios in SIM mode
- Verify tuning compatibility

Tony Taylor is the technical director of Crawford Scientific and ChromAcademy. He comes from a pharmaceutical background and has many years research and development experience in small molecule analysis and bioanalysis using LC, GC, and hyphenated MS techniques. Taylor is actively involved in method development within the analytical services laboratory at Crawford Scientific and continues to research in LC-MS and GC-MS methods for structural characterization. As the technical director of the CHROMacademy, Taylor has spent the past 12 years as a trainer and developing online education materials in analytical chemistry techniques.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)