The LCGC Blog: Underutilized Methods for Optimizing LC–MS Sensitivity
I hear the words “struggling for sensitivity” so often when speaking to folks using LC–MS for bioanalysis, environmental analysis, metabolomics, proteomics, and a host of other applications where target analytes are present at low concentrations in complex matrices. We spend fortunes on MS/MS instruments to increase specificity of detection in order to improve sensitivity. Some of us go to great lengths to optimize sample extraction and HPLC conditions in order to minimize matrix suppression effects and improve specificity and hence sensitivity.
I hear the words “struggling for sensitivity” so often when speaking to folks using LC–MS for bioanalysis, environmental analysis, metabolomics, proteomics, and a host of other applications where target analytes are present at low concentrations in complex matrices.
We spend fortunes on MS/MS instruments to increase specificity of detection in order to improve sensitivity. Some of us go to great lengths to optimize sample extraction and HPLC conditions in order to minimize matrix suppression effects and improve specificity and hence sensitivity. I’ve noticed a real trend in inquiries into the use of 1 mm i.d. columns in bioanalysis and metabolomics recently driven by the need to improve sensitivity of analyses.
We may optimize the mass analyzer for our target analytes by infusing solutions and adjusting various voltages to ensure good sensitivity. We select pre-cursor and product ions for MRM multiple reaction monitoring (MRM) experiments in order to produce the best signal-to-noise possible.
However, I wonder how many of us actually bother to optimize the conditions within the MS interface? I tend to refer to these parameters as “lock and leave.” That is, we optimized the various interface parameters when we first got the instrument and haven’t really touched them since-because we know that instrument X always run best with capillary voltage Y, nebulizing gas pressure Z, etc.
The key questions are, how much sensitivity could we gain by making time to optimize the interface parameters and why don’t we do this on a more regular basis?
To properly address these questions, we need to know a little about the processes which occur in the Atmospheric Pressure (API) Interface and here we will only consider the processes which are in play when using electrospray ionization (ESI).
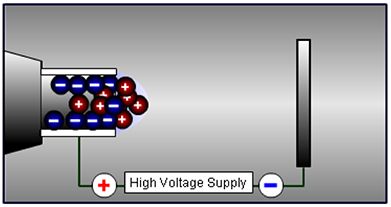
1. The HPLC eluent flows into a capillary nebulizer (sprayer), which is held under high-potential difference, causing ions of opposite charge to be attracted towards the walls of the capillary and those of like charge to remain within the eluent flow. The charge on the capillary is selected to match the charge on analytes of interest, or to rapidly switch between positive and negative modes.
Figure 1: Electrophoretic separation of charged analytes in the ESI sprayer.
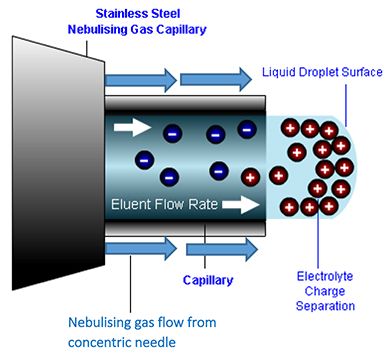
2. The eluent emerges at the capillary tip where it accumulates into a droplet, which will ultimately be released into the desolvation zone when the repulsion between charged analyte and matrix species at the droplet surface overcomes the liquid surface tension. It is important that the droplet is held at the capillary tip long enough to accumulate a significant number of charged analytes in a relatively small droplet volume, which at higher eluent flow rates (circa 0.5 to 1.0 mL/min) is assisted using a nebulizing gas, which effectively constrains the “growth” of the droplet whilst it accumulates charge. Higher eluent flow rates require higher nebulization gas flows and temperatures.
Figure 2: Charged droplets are formed at the capillary tip prior to release into the desolvation zone.
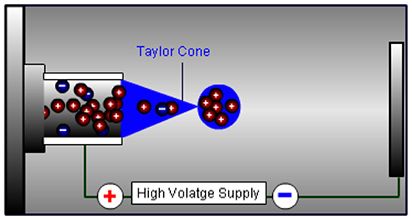
Figure 3: Nebulizing gas is used to constrain the growth of the droplet so as to ensure an optimum amount of charge per droplet.
3. Once the droplet leaves the sprayer, as it has accumulated a vast excess of either cationic or anionic analyte ions (depending upon whether the instrument is operated in positive or negative ion mode), it travels under the influence of the applied potential difference between the sprayer and sampling orifice.
4. The magnitude of the applied potential difference between the sprayer and sampling plate will determine the stability and efficiency of the “electrospray” and will have an “optimum” value depending upon the nature of the analytes and the eluent system.
5. As the sprayed droplets travels through a desolvation zone between the sprayer and the sampling orifice, the solvent will evaporate under the influence of a drying pas which is introduced into the API interface. The reducing droplet diameter brings ions nearer the solvent surface into closer proximity which causes an increase in electrostatic repulsion. Once these repulsive forces are great enough to overcome the droplet surface tension, the droplet undergoes jet fission, producing droplets of significantly lower volume, containing much higher charge concentrations. These offspring droplets rapidly undergo further fission events until, ultimately, either all of the solvent evaporates leaving gas phase ions or the droplets are small enough for ions to escape from the droplet under thermodynamically favorable conditions.
Figure 4: A drying gas is introduced into the desolvation zone to aid solvent evaporation from the sprayed droplets.

Figure 5: Subsequent droplet jet fissions occur until the droplet volume and number of charges create suitable conditions for analyte ions to be liberated into the gas phase. The position within the interface at which this occurs is vital to ensure maximum sensitivity.
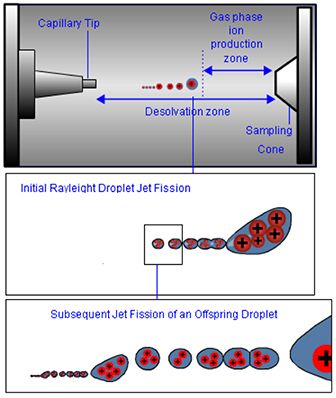
6. The position within the interface at which the gas phase ions are produced is very important, and ideally should be at the point at which the applied potential and vacuum within the first region of mass analyzer, are able to sample as many gas phase analyte ions as possible via the sampling orifice-the so called “sweet spot” for ion sampling.
The position at which the gas phase ions are produced within the desolvation zone is a product of the relative positions of the sprayer and sampling plate as well as all of the various parameters we have described above which include:
- Capillary (sprayer) voltage
- Nebulizing gas temperature and flow rate (pressure)
- Drying gas temperature and flow rate (pressure)
It is vital to realize that the settings required to obtain maximum analyte response for each variable will change with analyte type as well as the eluent, eluent additives and flow rate.
Perhaps this is a good time to pose our initial question again-“when was the last time you optimized any of these parameters?”
Two recent publications have helped to highlight the importance of these parameters:
- Optimization of the Electrospray Ionization Source with the Use of the Design of Experiments Approach for the LC–MS-MS Determination of Selected Metabolites in Human Urine. O. Szerkus, A. Yumba Mpanga, M. J. Markuszewski, R. Kaliszan, D. Siluk, Spectroscopy, Special Issues, 14(1), 8–16 (2016).
- Optimization of Electrospray Ionization by Statistical Design of Experiments and Response Surface Methodology: Protein–Ligand Equilibrium Dissociation Constant Determinations. L. Pedro, W.C. Van Voorhis, and R. J. Quinn, J Am Soc Mass Spectrom.,27 1520–1530 (2016).
The first publication highlights the simple optimization of source parameters using a one variable at a time (OVAT) approach in which two factor interactions are not considered. Even with this simple approach, two-to three-fold improvements in instrument response were realized. Further, it was clearly shown that the optimization of the two metabolite responses studied resulted in different values in both and negative ion modes. Applying a full factorial analysis of variance (ANOVA) treatment using a Box-Behnken design (BBD), three of the tested factors (drying gas flow rate, gas temperature, and nebulizer pressure) were found significant as well as two-factor interaction between gas flow and gas temperature, which had the highest influence on the response, and quadratic interaction of nebulizer pressure and gas temperature.
Again, we can ask ourselves the question, when was the last time we considered altering the drying gas flow rate or temperature to optimize the response from our LC–MS system?
Publication 2 above, also found drying gas temperature and flow rate to be significant variables in the response optimization of two different ligand complexes of Plasmodium vivax guanylate kinase (PvGK).
This study goes further and also considers a response surface approach for the optimization of selected mass analyzer voltages.
While optimization using design of experiments may not be available in every laboratory, simple OVAT approaches can be achieved using Microsoft Excel and the Solver plug-in which ships with every copy!
Next time you really need to optimize instrument response, consider changing the drying gas flow rate and flow (pressure) when infusing your analyte and tuning your mass spectrometer voltages or selecting the correct MRM values. It will also be possible to adjust the capillary voltage and nebulizing gas pressure in the same way.
One important consideration is the solution in which your analyte is dissolved to undertake the tuning exercise. Due to the effects of the eluent on ionization processes, the diluent solution should match the eluotropic strength at which the analyte will elute during the analysis and should also contain any additives which will be present. If multiple analytes need to be studied which elute at different points within the gradient, it may be necessary to take an “average optimum” set of conditions to best suit your experimental requirements and sensitivity specifications.
When you need absolute sensitivity, taking some time to optimize these underutilized instrument settings may reap great rewards!

Tony Taylor is the technical director of Crawford Scientific and ChromAcademy. He comes from a pharmaceutical background and has many years research and development experience in small molecule analysis and bioanalysis using LC, GC, and hyphenated MS techniques. Taylor is actively involved in method development within the analytical services laboratory at Crawford Scientific and continues to research in LC-MS and GC-MS methods for structural characterization. As the technical director of the CHROMacademy, Taylor has spent the past 12 years as a trainer and developing online education materials in analytical chemistry techniques.
The LCGC Blog: Historical (Analytical) Chemistry Landmarks
November 1st 2024The American Chemical Society’s National Historic Chemical Landmarks program highlights sites and people that are important to the field of chemistry. How are analytical chemistry and separation science recognized within this program?
