The LCGC Blog: Solvent Choice for GC Injection – a Critical Method Variable!
A short treatment of what to consider when choosing the appropriate sample solvent so you can be better informed when developing, optimizing, transferring, or troubleshooting your GC methods.
I guess we all have our “go to” solvents for our work in GC, and most of the time this will be based on the availability of the solvent and the chemical nature of our samples. Polar samples (analytes) generally need a more polar solvent (methanol for example) and less polar analytes a less polar solvent (n-hexane or heptane for example). Sometimes we might even use an intermediate polarity solvent when we aren’t sure of the analyte composition, ethyl acetate is a popular choice in this instance.
I talk to many folks involved in GC and the conversations around sample preparation and the choice of ultimate sample diluent are almost always around the chemistry of the sample (matrix) or the analyte and the requirements to properly solubilize the sample.
But there are so many more issues to consider when choosing the appropriate sample solvent and setting some of our critical method variables. I’ve given a short treatment of these considerations in this installment so you might be better informed when developing, optimizing, transferring or troubleshooting your GC methods.
Sample Solvent (Diluent) and Injection Volume
The sample diluent has a direct effect on the amount of sample which can be injected into the GC under a given set of inlet conditions.
As the sample is rapidly introduced into the GC inlet via the autosampler syringe, there is a rapid (explosive) evaporation of the sample solvent which transfers the sample molecules into the as phase. The degree of expansion will depend upon the nature of the sample solvent (for example, its expansion co-efficient), the inlet temperature and pressure, which is dictated by the combination of the liner size and the carrier gas flow rate through the inlet.
If the sample size (the number of microliters of sample you chose to inject) is too large, and other operating variables are not favorable, the gas phase sample will exceed the volume of the inside of the inlet liner, and the gas phase sample will contact the underside of the septum and “spill” over into the carrier gas inlet and septum purge lines. As these lines are not heated (other than the portions directly inside or next to the inlet), then the gas phase sample will condense, leaving some of your analyte deposited within the pipework of your sample inlet system.
If one were to then inject a “blank” solvent, under the same circumstances, the same overfilling phenomenon would occur, and just a wave “laps” the seaweed and other detritus from a beach, some of the previously deposited sample components within the pipework may be drawn back into the inlet and make their way into the column. We would be presented with a mini version of the previous chromatogram which we describe as “carry-over” and which is a major cause of quantitative irreproducibility in capillary gas chromatography. Chromatographers call this phenomenon backflash (Figure 1).
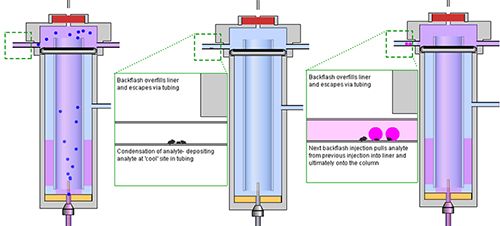
So – how does one decide on the proper injection volume or the operating variables which will avoid this insidious carry over effect?
Well, we typically use software calculators to assess the correct amount of sample to inject and the appropriate inlet temperature and pressure. The calculator needs to know the nature of the solvent and its expansion co-efficient in order to calculate the volume of gas created under a given set of conditions, from a given volume of solvent injected into the system. It will also need the liner volume (easily calculated or requested from suppliers) and the temperature and pressure of the inlet, which of course you can get from the GC system.
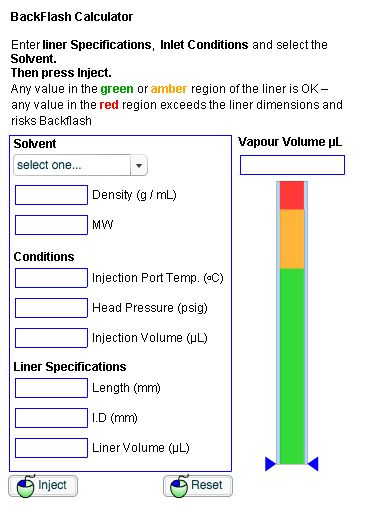
These calculators are very useful as they allow one to assess the required changes to the operating conditions in order to avoid backflash, or on a more positive note, to assess how much sample can be injected without risking carry-over, if one wants to improve the sensitivity of a method.
Further, the calculator can be used to make informed choices on the detector temperature setting required for a particular method, alongside the general information that one might have about the boiling points of the higher boiling sample components.
The calculator can also be used to assess the effects of increasing the head pressure (carrier gas flow), during the moment of sample injection which constrains the expansion of the sample solvent plug, which is known as pressure pulsed injection, a strategy which is also used to increase the volume of sample injected without risking backflash and hence analyte carry-over.
Diluent effects on peak shape
During the initial phase of a splitless injection, the column is cool (40–70 °C typically) and the sample solvent is allowed to condense on the stationary phase on the inner wall of the capillary column. Due to the reduced vapor pressure caused by the flowing carrier gas, the solvent evaporates and "concentrates" the dissolved analytes into a sharp band.
This effect, known as solvent focusing, helps to overcome the very broad peak shapes associated with splitless injection into a hot oven, caused by the prolonged time taken to move the sample vapor from the inlet to the column under splitless conditions.
The peak focusing effects rely upon the solvent forming a contiguous (uninterrupted) film on the inner wall of the capillary column. If the polarity of the column stationary phase is not well matched with the polarity of the sample diluent, this film formation can fail and "pooling" of solvent can occur which results in a non-contiguous film and very poor peak shape.
These effects along with the resulting chromatograms can be seen in Figure 3.
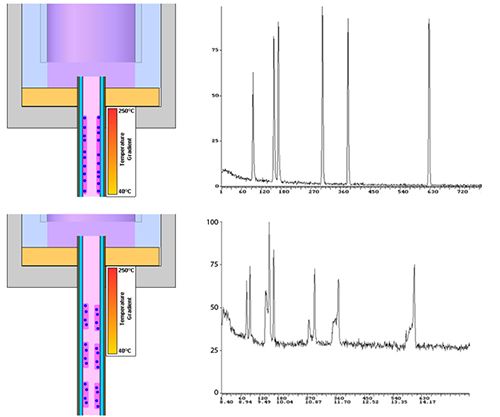
These effects can also occur to a lesser extent in Split injection, however they are typically much less severe.
Typically, one should avoid extremes such as using methanol as a sample diluent with non-polar GC stationary phases such 100% polydimethylsiloxane or n-hexane as a sample diluent when using polar phases such as Waxes. However, minor effects can also be seen when using intermediate polarity phases or solvents and one should take care to match the solvent polarity with that of the stationary phase.
So, the next time your reach for the solvent bottles to dissolve or reconstitute your sample prior to loading it into the GC vial – just stop a moment and run through the considerations above. Make an informed solvent choice – your chromatography will be all the better for it!

Tony Taylor is the technical director of Crawford Scientific and ChromAcademy. He comes from a pharmaceutical background and has many years of research and development experience in small molecule analysis and bioanalysis using LC, GC, and hyphenated MS techniques. Taylor is actively involved in method development within the analytical services laboratory at Crawford Scientific and continues to conduct research in LC-MS and GC-MS methods for structural characterization. As the technical director of the ChromAcademy, Taylor has spent the past 12 years as a trainer and developing online education materials in analytical chemistry techniques.
The LCGC Blog: Historical (Analytical) Chemistry Landmarks
November 1st 2024The American Chemical Society’s National Historic Chemical Landmarks program highlights sites and people that are important to the field of chemistry. How are analytical chemistry and separation science recognized within this program?

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)