The LCGC Blog: HPLC Diagnostic Skills—Systematic Investigation of Selectivity Changes
Following a checklist can help to diagnose changes in chromatographic selectivity, which can be among the most difficult to isolate and correct.
adzicnatasa/stock.adobe.com

Following a checklist can help to diagnose changes in chromatographic selectivity, which can be among the most difficult to isolate and correct.
The selectivity (α) of an analytical system describes the ability to discriminate between sample components based on differences in chemical and physicalâchemical properties. In chromatographic terms, it describes the spacing between the apices of the peaks within the chromatogram (Figure 1) and is determined not only by the analyte properties, but also those of the stationary phase and eluent system.
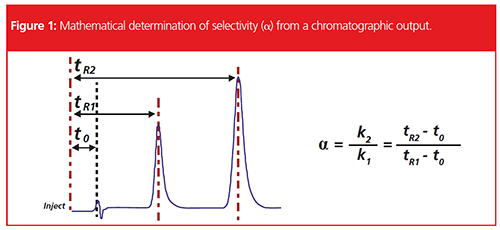
The selectivity obtained by an analytical method is fundamentally important to chromatographic separations and, in combination with peak efficiency (crudely, peak width), will determine the resolution of peaks within the chromatogram. When selectivity changes, resolution may decrease, making peak area measurement less accurate and reproducible, as well as decreasing confidence in peak identification or spectral structural elucidation.
Selectivity changes can range from subtle shifts in relative retention of a single peak within the chromatogram to wholescale retention order swapping of every peak, and we need to be able to spot these changes and investigate the causes in a systematic and proficient manner. However, with so many variables affecting selectivity, it is sometimes difficult to know where to begin our diagnostic investigations.
Figure 2 shows an example of a subtle change in chromatographic selectivity (peaks 1 and 2), which led to difficulties with accurate peak quantitation.
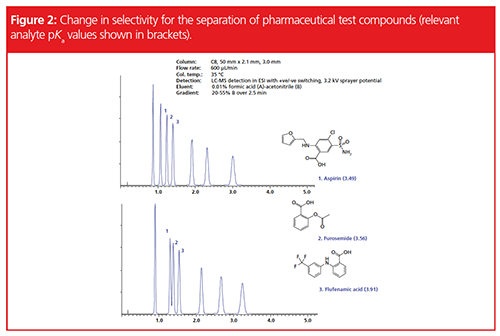
As we can see from Figure 2, the selectivity change is also accompanied by a slight shift to later retention, and when retention shift occurs, it’s a good idea to check the selectivity values for the peaks in question to confirm that a selectivity change has occurred. In this case it’s visually obvious that we have had a change to the chemistry of the system, and therefore we will go ahead and invoke the selectivity diagnostic checklist to investigate the issues.
It’s important to note that the checklist is in order of “ease and probably”, for example, things which are simple to check are included earlier in the checklist, to make our investigations as efficient as possible.
1. Check the gradient profile of the method-Check that the correct gradient profile has been entered into the chromatography data system (CDS) acquisition method. Enter the correct gradient profile if required and re-analyze the sample or standard solution.
2. Confirm correct stationary phase-The column stationary phase is obviously critical to the chemistry of the separation and our first diagnostic is to check we have the correct column (including the column dimensions). Note that it is very common for selectivity changes to occur when switching between brands or manufacturers, even if the nominal stationary phase chemistry (such as C18) is the same. Be sure to only use the prescribed phase and manufacturer
for the method, unless phase equivalence has been previously established and validated. Change to the correct column if required and re-analyze the sample and standard solution.
3. Check stationary phase batch and lot number against previous “good” separation-The selectivity of certain (usually older or more complex) stationary phases may change between batches or lots, due to subtle or uncontrollable changes in the manufacturing (bonding) chemistry. It is also possible for selectivity changes to occur if different column hardware (tubing or frits, and so on) are used. Verify the batch and lot number and, if different, begin the hunt for a column from the same batch, but do not change the column at this point.
4. Verify correct column temperature-Changes in column (eluent) temperature can affect the selectivity of separations, and this can be more noticeable when dealing with ionogenic analytes (as is the case in our example above). Ensure that the correct column compartment temperature is selected in the CDS and that the correct temperature is established in the column heater using a thermal resistance thermometer (RTD). It is sometimes necessary to adjust the selected column compartment temperature to obtain the correct temperature value as measured using the RTD. Also worthy of note here are the differences between the eluent pre-heating option between various manufacturers. Some systems flow the eluent through a small void volume within the metal fabric of the column compartments to pre-heat the eluent to the required column temperature. Even small differences in the volume of the pre-heat section hydraulic path can affect selectivity in very sensitive separations, and so differences between high performance liquid chromatography (HPLC) system manufacturers should be borne in mind. Ensure the correct column and eluent temperatures are set and achieved and re-analyze the sample or standard solution. A “last resort” check when temperature is suspected as the cause of selectivity differences is to switch the separation to a system known to give good chromatography with the method under investigation.
5. Check mobile phase pH-The pH of the eluent can make a big difference to the selectivity of a separation when analyzing ionogenic analytes and in certain cases, a very small change in the eluent pH can result in large selectivity differences. Check using a calibrated pH meter bearing in mind the best practice approaches for pH meter usage. Ensure that the pH is correct to within two decimal places or at least the number of decimal places specified in your method. Bear in mind that when using volatile mobile phase additives (formic acid, trifluoroacetic acid, ammonia, and so on) that the additive may be lost over the course of a long campaign of analysis and therefore pH may “creep” over time. Try to avoid mistakes with eluent preparation by specifying if pH modifying additives are to be added in weight to volume (w/v) or volume to volume (v/v) fashion (such as 0.1% formic acid v/v rather than just 0.1% formic acid). It is beyond the scope of this blog entry, however, it is best policy, and most accurate, to prepare eluent systems gravimetrically; for more information see reference 1. Check and correct the eluent pH-combined with Check 6 prior to reâanalysis.
6. Check the eluent eluotropic strength and buffer concentration (2)-Ensure that the correct organic modifier solvent has been used and the correct solvent channels selected in the CDS method. Where mobile phases are pre-mixed, ensure that each volume is measured separately before mixing, to avoid volume changes or inaccuracies due to latent heat of mixing. This is especially true when using methanol as the organic modifier. Ensure that the correct buffer salt, pH modifier, or additive has been used (for example, disodium hydrogen orthophosphate does not have the same chromatographic properties as sodium dihydrogen orthophosphate) and that the correct weights or volumes of the additives have been used. As previously stated, one needs to check if additives are specified as volume to volume or weight to volume. Once these checks have been carried out or a fresh eluent has been prepared, re-analyze the sample or standard solution and check the selectivity. If you managed to get an HPLC column from the same batch or lot number as the original column, then this would be a good time to try the new column and assess
the selectivity.
Again, note that any volatile eluent additives may evaporate on standing and this may affect both retention and selectivity over long analytical campaigns or between batches where the eluent has been allowed to stand on the instrument.
7. Check dwell volume differences between instruments-In gradient HPLC, differences in the way that the eluents are mixed (binary versus quaternary pumping systems) or differences in the system volume between the point at which the eluent is mixed to the head of the analytical column can cause differences in retention time and selectivity of the separation. When switching between HPLC systems for a particular method, one needs to verify that these inherent system differences have been accounted for; this is usually achieved by adding an isocratic hold to the initial portion of the gradient or even by introducing an injection delay, whereby the sample is injected at some time after the gradient has started. The details of the underlying theory and corrective actions can be found in reference 3, however, this problem is more typical than is usually thought, and as such, if all other checklist items have been implemented then switching the column and eluent system to a system on which the last satisfactory separation was achieved will be indicative of dwell volume difference issues. Use reference 3 to adjust the gradient characteristics and re-analyze the sample and standard solution.
8. Verify gradient mixing and delivery accuracy and capability-Sometimes instruments get a little worn out and their capacity to mix the gradient in an accurate or reproducible fashion diminishes. In this instance, trying a different instrument is probably the simplest solution in the short term. Quaternary pumps, which mix the gradient using a series of electronically actuated valves on a “time” basis are more prone to gradient mixing inaccuracy than binary pumps, which deliver the correct eluent composition based on differential flow or piston stroke volume. There are various tests to identify the effectiveness of the quaternary mixing valve, but the simplest is to dope the eluent (channel B) with 0.1% v/v acetone and measure the UV response at 268 nm during a gradient which is programmed in “steps” of 20% per segment, for example, 10% B for 5 min, 10% B per min until 7 min, hold for 5 min at 20% B, and so forth. Replace the mixing valves if necessary, noting that sometimes eluent mixing issues can be caused by cavitation induced by blockages in the sinker and filters within the eluent reservoirs. These should also be cleaned as necessary, following your manufacturer’s recommended cleaning process, on a regular basis.
Sometimes we are a little overambitious when programming ballistic gradients, those which rise over a large range of eluent B in just a few minutes or even seconds (it can be argued the current method under investigation falls into this category). Unless we are using the highest efficiency, lowest dead-volume pumping systems, then the instrument may not be to able to accurately or reproducibly deliver the required gradient. Again, a detailed treatment of this topic is beyond the scope of our discussion here, but reference 4 contains more details on this topic.
In our example, we followed the checklist to item number 5 and found that there was an error in the eluent preparation, which had been prepared volumetrically rather than gravimetrically. A 0.01% measure of formic acid prepared volumetrically had resulted in an aqueous solution pH of 3.22, whereas the gravimetric method resulted in a solution pH of 3.27. While this difference of 0.05 pH units may seem vanishingly small and inconsequential, when the eluent pH was so close to the pKa value of the analytes of interest, this had resulted in the change of chromatographic selectivity. The separations obtained from the freshly made eluent, compared to the last time the method was run, are shown in Figure 3. The further point to be made here is that, in as far as is possible, methods should be designed with the eluent pH at least 1 (and preferably 2) pH units away from the pKa values of the analytes of interest. Undoubtedly the analyst who developed this method used fine control of pH to finesse this rather difficult separation, however, perhaps a better route would have been to have the analytes in their fully ionized or non-ionized forms and sought an alternative column chemistry and organic modifier to obtain a satisfactory separation. Reference 5 contains more details on this type of alternative approach to method development.
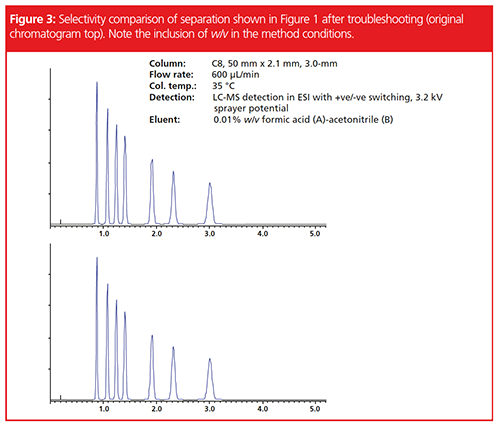
It should be noted that we also studied the gradient delivery performance for the instrument used and the accuracy and reproducibility of the gradient profile were satisfactory, so this was ruled out as a potential issue.
In summary, changes in chromatographic selectivity can be among the most difficult to diagnose and correct. One should properly establish that selectivity has changed, and implementation of the diagnostic checklist outlined above represents a logical and time-efficient way in which to isolate and ultimately correct the problems with the chemistry of the separation. Wherever possible, chromatographic separations should be designed with robustness in mind, and where this is not possible, method specifications should be carefully written to highlight the key variables within the separation conditions.
References
- D.R. Stoll and D.M. Makey, LCGC Europe32(7), 364–369 (2019).
- T. Taylor, LCGC North America33(12), 922 (2015).
- T. Taylor, The LCGC Blog: http://www.chromatographyonline.com/lcgc-blog-dwell-volume-still-relevant-our-uhplc-world
- T. Taylor, The LCGC Blog: http://www.chromatographyonline.com/lcgc-blog-hplc-gradients-getting-it-right-issues-hplc-gradient-reverse-phase-chromatography
- T. Taylor, The LCGC Blog: http://www.chromatographyonline.com/lcgc-blog-generic-methods-potluck-supper-analytical-chemistry
Tony Taylor is Group Technical Director of Crawford Scientific Group and CHROMacademy. His background is in pharmaceutical R&D and polymer chemistry, but he has spent the past 20 years in training and consulting, working with Crawford Scientific Group clients to ensure they attain the very best analytical science possible. He has trained and consulted with thousands of analytical chemists globally and is passionate about professional development in separation science, developing CHROMacademy as a means to provide high-quality online education to analytical chemists. His current research interests include HPLC column selectivity codification, advanced automated sample preparation, and LC–MS and GC–MS for materials characterization, especially in the field of extractables and leachables analysis.
E-mail:tony@crawfordscientific.comWebsite:www.chromatographyonline.com
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




