The LCGC Blog: Avoiding the Problems Associated with HPLC Column Overload
Much has been written about column overload, however, I've seen many instances lately in which overload may not be instantly recognized, or causes problems that may not normally be attributed to overload. Therefore, I wanted to explain how to spot and deal with different types of overload situations.
Much has been written about column overload, however, I've seen many instances lately in which overload may not be instantly recognized, or causes problems that may not normally be attributed to overload. Therefore, I wanted to explain how to spot and deal with different types of overload situations.
Gross overload tends to lead to peaks which either front or tail badly and whose retention times may vary from the non-overloaded separation. However, more subtle overload effects are more insidious and can be seen in everyday chromatography as shown in Figure 1.
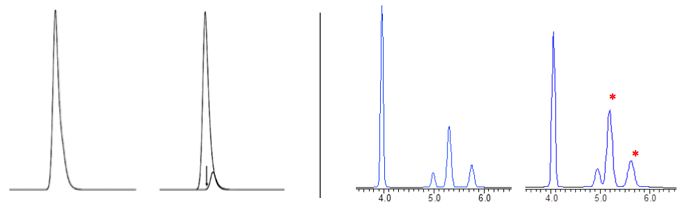
Figure 1
(Left): Tailing peak due to overload conditions (left) or an unresolved impurity (right)? The distinction between the two may require a different set of conditions or a spectral detector (1)
(Right): Reversed phase separation in which loading is doubled from 10µg to 20µg which effects resolution and ease of integration for the two peaks at around 5mins. (note * denotes ionizable compounds)
There are two different types of overload –
The physical symptoms of each of these overload situations are different.
In Mass (sometimes also called Concentration) Overload the analyte molecules "saturate" the silica at the inlet end of the column which causes the excess molecules to flood forward down the column, which shifts the centre of mass of the analyte band down the column, and results in a right sided "shark fin" type peak and a dramatic reduction in the apex retention time as shown in Figure 2.
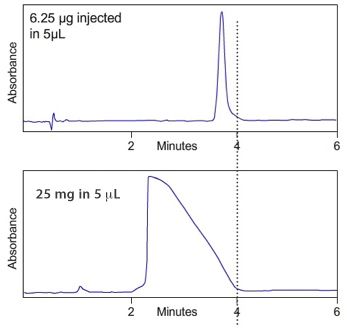
« Figure 2:
(Top); Gross mass overload of a neutral analyte under reversed phase conditions which shows a drastic reduction in retention time and a "shark fin" type peak (Figure courtesy of YMC Europe GmbH, Dinslaken, Germany.)
It is generally accepted that for neutral compounds being separated using hydrophobic stationary phases in reversed phase HPLC, absolute sample loadings (i.e. amount of sample injected onto the column) of between 1 and 10 mg should be possible. Table 1 below shows the maximum theoretical load estimates for a range of popular column dimensions, based on the assumption that a 4.6 mm i.d. column contains around 1g of silica packing material per 10 cm of column length and a 2.1 mm i.d. column contains around 0.2 g of silica per 10 cm of column length.
150 x 4.6
100 x 4.6
50 x 4.5
100 x 2.1
50 x 2.1
30 x 2.1
15
10
5
0.2
0.1
0.06
Table 1: Loading estimates for
some common column dimensions.
The mass loading estimates are a very crude approximation and empirical measurements should be made if mass overload is suspected. Generally one would reduce the mass injected by factors of 10 (or 2 for trace analysis) until the retention time and peak symmetry stabilize.
When moving from one column to another with different surface area and pore volumes, one can use the following approximation (eqn. 1) for the phase ratio of a packed bed (ratio of silica surface to eluent) to assess the loadability of the new column.
P = Asp/ (Vsp +Ei/(1-Ei)*(Vsp+1/ρ)) (eqn. 1)
Asp – surface area of packing (m2/g)
Vsp – specific pore volume (mL/g)
Ei – interstitial fraction (typically 0.4)
ρ - silica density (2.2 g/mL for fully porous silica)
So – if a loading for Column 1 is found to be 25 µg, what will be the loading for Column 2 as shown below?
Column 1
P = Asp/ (Vsp +Ei/(1-Ei)*(Vsp+1/ρ))
P = 380/ (1 +0.4/(1-0.4)*(1+1/2))
P = 380/ (1 +0.4/(1-0.4)*(1+1/2)) = 109
Column 2
P = Asp/ (Vsp +Ei/(1-Ei)*(Vsp+1/ρ))
P = 180/ (0.4 +0.4/(1-0.4)*(1+1/2))
P = 180/ (0.4 +0.4/(1-0.4)*(1+1/2)) = 112.5
Therefore – the loadability of Column 2 is approximately the same as Column 1 (25 mg)
Everything we have considered to this point assumes that the analytes are in the neutral state. When dealing with ionized analytes, loading factors may reduce by a factor of between 10 and 50 times when using conventional reversed phase stationary phases.
This is demonstrated in Figure 3, where Quetiapine, an ionized basic analyte under acidic conditions, shows gross overloading whereas the neutral analyte propiophenone, does not suffer from the same degree of overloading.
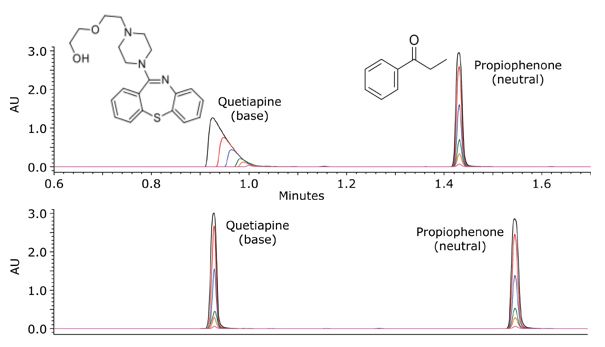
Figure 3; Loading capacity for quetiapine (base) and propiophenone (neutral) in acidic conditions (pH~2.7) on a fully porous C18 column. Mobile phase A was 0.1% formic acid in water, and mobile phase B was 100% acetonitrile. The gradient was from 5 to 95% B in 2.5 min. Flow rate was 0.6 mL/min. UV detection at 254 nm. Injection volume was 5 μL. Column temperature was 30 °C. Column dimensions were 2.1 x 50 mm, 1.7 μm. The mass load on-column was between 10 ng (green chromatogram) and 1 μg (orange chromatogram) for each compound. (2)
The stationary phase surface becomes associated with charged analytes which are retained via hydrophobic interactions. The positive nature of the surface effectively 'repels' the positive charge of analytes in the eluent, and this phenomenon is particularly pronounced within the stationary phase pores, where analytes can be ion-excluded, thus effectively reducing the apparent column surface area.
There are a number of ways of dealing with this issue, including the obvious approach of adjusting the eluent pH to ensure the analyte is in the ion-supressed form. However modern HPLC columns are available with mixed mode stationary phases, which contain electrostatic groups of opposite charge to the analyte, which help to reduce the electrostatic repulsion effects described above.
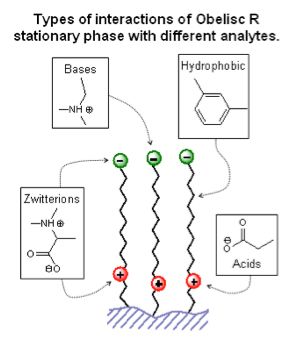
« Figure 4; Example of a zwitterionic mixed mode stationary phase which can interact both hydrophobically and electrostatically, which helps to reduce ion-exclusion and improve loadability of ionized compounds (Figure courtesy of SIELC Technologies, Prospect Heights, IL, USA.)
Figure 5 shows a loading study with dopamine on a reversed phase C18 column which is marketed as providing good retention of polar compounds and a zwitterionic mixed mode phase. Note that the retention time and peak shape on the mixed mode phase are affected to a much lesser degree than the standard stationary phase
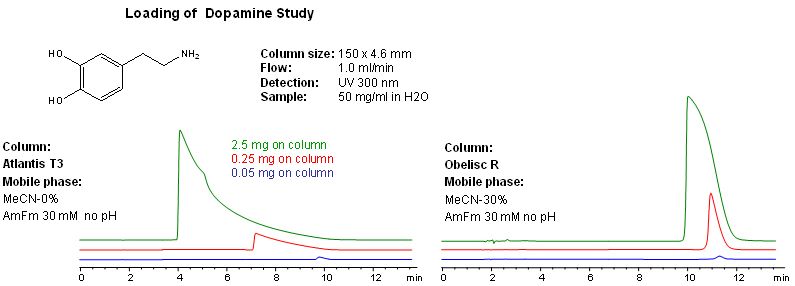
Figure 5: Ionized analyte loading study using a mixed mode and non-mixed
stationary phase (Figure courtesy of SIELC Technologies, Prospect Heights, IL, USA.)
Volume overload occurs when the injected sample volume is large enough to carry analyte molecules through a
significant proportion of the interstitial volume within the column and leads to shark fin type peaks and later elution
times as shown in Figure 6.
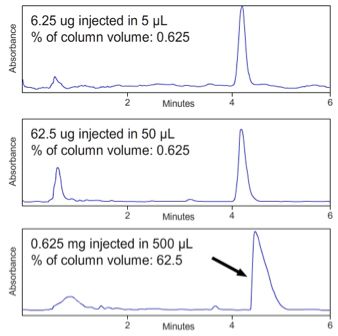
« Figure 6; Volume overload at constant sample concentration on a C18 reversed phase HPLC column (150 x 4.6 mm, 5µm) (Figure courtesy of YMC Europe GmbH, Dinslaken, Germany.)
Volume overload can be avoided if the injection volume is kept below 15% of the peak volume. We can ESTIMATE this for a range of columns of various dimensions given some theoretical efficiency (plate) values.
Table 2: Target efficiencies for some common HPLC dimensions
N = 16 (tr/wb)2 (eqn. 2)
Equation 2 can be re-arranged to allow an estimation of the peak width (volume);
wb = tr / √(N/16)
So, for a peak eluting at 2.5 min on a 50 x 2.1 mm column;
wb = 2.5 / √(5000/16)
wb = 0.14 min.
If the column flow rate is 0.5mL/min then the peak volume will be
0.14 x 0.5 = 0.070 mL
and 15% of this volume is;
0.070 x 0.15 = 0.011 mL = 11µL
Finally, the strength of injection solvent used to dissolve the sample can have a pronounced effect on the loadability of analytes.
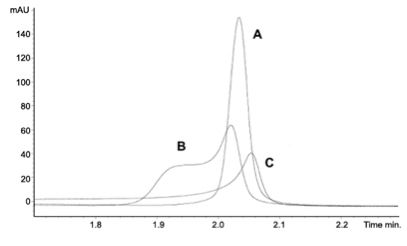
« Figure 7: Peak shape effects on a caffeine sample dissolved in (A) 33% acetonitrile (B) 66% acetonitrile and (C) 100% acetonitrile. Caffeine at 0.75 mg ml−1, 4 μl injection volume. Column: Luna 3 μm C18(2) 50×2.0 mm. Mobile phase: gradient 5 to 95% acetonitrile (with 0.1% formic acid) in 7.5 min at a flow-rate of 1 ml min−1. (Adapted from Reference 3.)
For more information – contact either Bev (
bev@crawfordscientific.com
) or Colin (
colin@crawfordscientific.com
).
For more tutorials on LC, GC, or MS, or to try a free LC or GC troubleshooting tool, please visit www.chromacademy.com
The LCGC Blog: Historical (Analytical) Chemistry Landmarks
November 1st 2024The American Chemical Society’s National Historic Chemical Landmarks program highlights sites and people that are important to the field of chemistry. How are analytical chemistry and separation science recognized within this program?

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)