The LCGC Blog: Allowable Changes to Pharmacopeial HPLC Methods
I started my career working in a Quality Control laboratory. I'm familiar with the constraints that must be imposed to ensure consistent global standards and the pace at which change can be implemented without loss of control either within a business or across an industry. OK-scene set.
I started my career working in a Quality Control laboratory. I'm familiar with the constraints that must be imposed to ensure consistent global standards and the pace at which change can be implemented without loss of control either within a business or across an industry. OK-scene set.
However, I really feel it's time for the Pharmacopeia to drag themselves into the 21st Century and realize the benefits of using smaller diameter stationary phase particles and superficially porous particle morphology. Higher resolution and higher throughput can both be realized by these approaches, which can be very beneficial in a QC laboratory environment, yet many pharmacopeial methods still use long columns with large (5 mm) fully porous particles.
Whilst I'm sure many of you will be aware of what changes are possible to pharmacopeial methods, I still see many old or under developed methods which could be updated and I'm often asked what changes can and can't be made-hence I've written this so I can point folks to it for reference!
The big change to allowable method adjustments came in August 2014 when USP37-NF32S1 was released and Chapter <621> Chromatography set-out a revised schedule of allowable changes to methods. For HPLC methods I've outlined the changes in Table I below with some further explanation of how the changes have been interpreted.
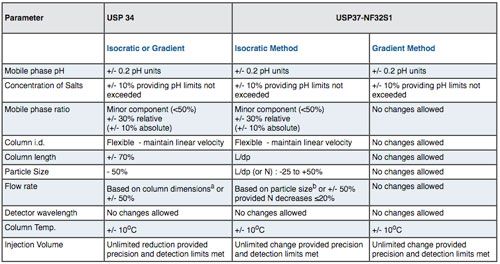
CLICK TABLE TO ENLARGE
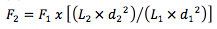
[eqn. 1] F – flow rate, L- column length, d – column diameter
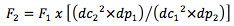
[eqn. 2] F – flow rate, dc- column internal diameter, dp – particle diameter
Some notes and comments on the new allowable changes to help interpret them and highlight issues and difficulties.
Of course, scaling to this extent will need some adjustments in flow rate which can be calculated using Equation 2 above;
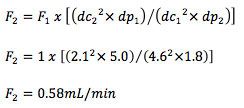
This change is within the allowable +/- 50% range from the original 1mL/min. flow rate but we need to also ensure that the efficiency of the peaks does not reduce by more than 20%.
With efficiency in mind, one must be very mindful of the requirements for lower extra column volume within the system, and it will be likely that a different HPLC system will be required with lower extra column and flow cell volumes and the ability to cope with higher back pressures.
We can also approximate the new injection volume using the equation;
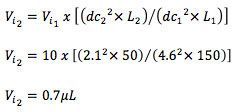
It's important here to note that here we have used the calculations above to give us an “equivalent” separation within approximately the same time frame. One may wish to increase the flow rate in order to obtain a faster separation and we would therefore need to carefully check system back pressure is within the allowable range for the instrument and that the USP37-NF32S1 guidelines of F1 +/- 50% and N decreases ≤20% are met.
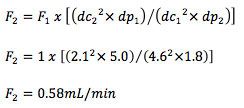
This change is within the allowable +/- 50% range from the original 1mL/min. flow rate but we need to also ensure that the efficiency of the peaks does not reduce by more than 20%.
With efficiency in mind, one must be very mindful of the requirements for lower extra column volume within the system, and it will be likely that a different HPLC system will be required with lower extra column and flow cell volumes and the ability to cope with higher back pressures.
We can also approximate the new injection volume using the equation;
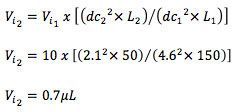
It's important here to note that here we have used the calculations above to give us an “equivalent” separation within approximately the same time frame. One may wish to increase the flow rate in order to obtain a faster separation and we would therefore need to carefully check system back pressure is within the allowable range for the instrument and that the USP37-NF32S1 guidelines of F1 +/- 50% and N decreases ≤20% are met.
- Please note the (severe) restrictions on gradient HPLC methods. Adjustments to methods which take advantage of smaller or superficially porous particles are restricted to isocratic methods only. Again, while being sympathetic to the need for control, I do wonder why there have not been more efforts to specify allowable changes to gradient methods.
- For clarification on the mobile phase strength guidelines;
An eluent containing 60:40 aqueous:organic can be changed in the following ways –
A 30% change to the 40% organic is 12%, however this exceeds the 10% absolute limit of change allowed-therefore the range of allowable change for this eluent system would be 20% - 50% i.e. +/-10% absolute.
An eluent containing 90:10 aqueous:organic can be changed in the following ways:
A 30% change to the 10% organic is 3.0%, which does not exceed the 10% absolute limit of change allowed-therefore the range of allowable change for this eluent system would be 7.0 – 13.0% i.e. +/-30% of the original organic %.
- The L/dp ratio can be explained using the Equation 3;
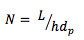
For small molecules separated using well packed columns with fully porous particles, the reduced plate height will have a value of around 2 (1). This constant for the reduced plate height allows us to use the column length to particle size ratio (L/dp) within the range of 25% to +50% of the original value.
So an original isocratic method using a 150 x 4.6mm column with 5μm particles (L/dp = 30,000) with a flow rate of 1mL/min and an injection volume of 10μL can be scaled to a column dimension of
50 x 2.1mm with 1.8μm particles (L/dp = 27,778)
This represents a change in L/dp of approx. -7.4%, which is allowable under the USP37-NF32S1 guidelines.
Of course, scaling to this extent will need some adjustments in flow rate which can be calculated using Equation 2 above;
- For superficially porous particles, the reduced plate height value can change to values between 1.4 and 1.6 and so we will obtain a marked increase in the value for N according to Equation 3 above and in these circumstances we need to take care not to EXCEED the range of values given for “equivalent N” in the USP37-NF32S1 guidelines (-25 to +50% of original N value). An example for the method translation of a Naproxen and related substance assay are shown below, where various L/dp combinations of core-shell columns are tried without any other adjustments to the method conditions;
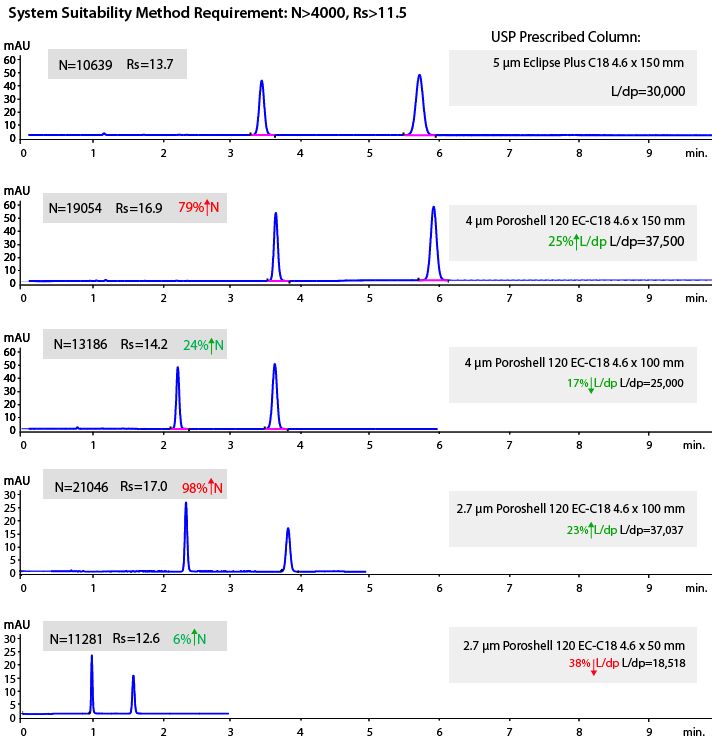
One should recognize that several of these chromatograms produce excellent separations but which are outside the allowable range USP37-NF32S1-and these “transgressions” in either L/dp ratio or efficiency (N) are highlighted in red. However, it should also be obvious that by choosing a 100 x 4.6, 4μm core-shell column and changing no other parameters, the guidelines are met and the overall run time is halved.
So, I've shown here that we can take a pragmatic or empirical approach to changing isocratic HPLC methods from the USP to take advantage of modern particle technologies. Let's hope to see a set of guidelines which highlight allowable changes to gradient HPLC methods soon! As a foot note-please just be aware that these changes are for USP methods only and that other Pharmacopeial authorities may differ in the changes which are allowed.
Reference
- L. R. Snyder, J. J. Kirkland, and J. W. Dolan, Introduction to Modern Liquid Chromatography, 3rd ed.; John Wiley & Sons: U.S., p. 205, (2010).

Tony Taylor is the technical director of Crawford Scientific and ChromAcademy. He comes from a pharmaceutical background and has many years of research and development experience in small molecule analysis and bioanalysis using LC, GC, and hyphenated MS techniques. Taylor is actively involved in method development within the analytical services laboratory at Crawford Scientific and continues to conduct research in LC-MS and GC-MS methods for structural characterization. As the technical director of the ChromAcademy, Taylor has spent the past 12 years as a trainer and developing online education materials in analytical chemistry techniques.
The LCGC Blog: Historical (Analytical) Chemistry Landmarks
November 1st 2024The American Chemical Society’s National Historic Chemical Landmarks program highlights sites and people that are important to the field of chemistry. How are analytical chemistry and separation science recognized within this program?
