LC-MS-MS Analysis of Antibiotics in Wastewater
This article describes the development of a method for the determination of sulphonamide antibiotics at trace levels in effluent wastewaters by LC?MS?MS to determine its capability for the detection of pharmaceutical compounds before they reach the aquatic environment.
Introduction
Antibiotics are widely used in human and veterinarymedicine for the prevention and treatment of bacterialinfectious diseases. An important but oftendisregarded aspect of antibiotic use is the fate ofantibiotic residues entering the environment.1Pharmaceutical industry wastewater, improperlydisposed of unused antibiotics and non-metabolizedantibiotics excreted by humans can all enter the sewersystem in low concentrations. Because sewagetreatment plants are rarely equipped to remove thesedrugs from wastewater, antibiotics are released intothe water system where they can enter theenvironment and eventually the drinking water supply.Veterinary antibiotics used in livestock operations areanother major source of contamination in theenvironment. Agricultural waste such as manure andwater run-off can carry these antibiotics into the soiland groundwater.
The effects of antibiotics in the environment are stillpoorly understood. One major concern is thedevelopment of antibiotic-resistant strains of bacteriathat could critically disturb the natural bacteriaecosystems and lead to a serious threat to human health. There are also concerns that exposure to environmentalantibiotic residues might lead to carcinogenic or allergicreactions in humans and create hazards to aquatic and soil organisms.2,3
The presence of antibiotics and other drugs in theenvironment has garnered international attention recently. TheAssociated Press (AP) reported in March 2008 that a variety ofprescription and over-the-counter drugs have been found inthe drinking water supplies of at least 41 million Americansliving throughout the US.4 Achieving low limits of detection(LODs) of pesticides, antibiotics and veterinary residues indrinking water is of paramount importance to monitor theregulatory levels as stated by US, Canadian, Japanese andEuropean environmental and water directives. Because many ofthese substances may pose a significant health threat, theyneed to be accurately detected.
Traditionally, liquid chromatography coupled with tandemmass spectrometry (LC–MS–MS) has been used by theenvironmental industry for the identification and quantificationof these residues. This methodology typically requires extensiveoff-line sample preparation. Additionally, the compounds ofinterest are generally present at trace levels, so the samplepreparation method requires preconcentration. Researchershave recently developed an on-line preconcentration methodfor sample preparation of water samples that overcomeschallenges related to sample preparation of water samples.5
Sulphonamides (Figure 1) are a common class of syntheticantimicrobials that are widely used in human and veterinarymedicine and as feed additives to promote growth inconcentrated animal feeding operations. They are regarded asemerging contaminants that are introduced into theenvironment predominantly in the US and Europe. There is noregulation of the levels of these compounds in environmentalmatrices (water, sediment, soil). This is probably because of thelimited knowledge of the input, fate and effects of mostpharmaceuticals in the environment. Therefore, sensitive andreliable analytical methods for detection of low concentrations(ng/L) of these compounds are needed.
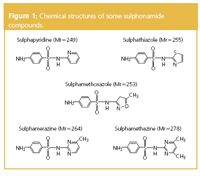
Figure 1
Experimental Conditions
Sample preparation: Samples of secondary effluent werecollected from sewage treatment plants in Greece and thenvacuum filtered. Each 50 mL sample was diluted with 200 mLdeionized water. After acidification to pH 4, 5 ng of thesurrogate standard d4-sulphamethoxazole (d4-SMX) wasadded before enrichment to assess possible losses during the analytical procedure. The effluent samples were enriched bysolid-phase extraction (SPE). The diluted wastewater sampleswere percolated through the cartridges at a flow-rate of5 mL/min. The cartridges were then washed with 5 mLdeionized water. Wastewater organics were eluted with 2 X4 mL methanol. The solvents were evaporated under a streamof nitrogen gas and then the extracts were redissolved in0.5 mL mobile phase A (0.1% formic acid in water).6 While thismethod employed the more traditional off-line method ofsample preparation, other researchers have successfullyemployed an on-line preconcentration method for samplepreparation.5
HPLC: HPLC analysis was performed using the Surveyor HPLCsystem (Thermo Fisher Scientific, San Jose, California, USA).Each 20 μL sample was injected directly onto a 150 3 2.1 mm,3.5 μm, C18 HPLC column. A gradient LC method used mobilephases A (0.1% formic acid in water) and B (0.1% formic acidin acetonitrile) at a flow-rate of 0.2 mL/min.
MS: MS analysis was performed on a TSQ Quantum Ultra triplestage quadrupole mass spectrometer with an electrosprayionization source (Thermo Fisher Scientific, San Jose, California,USA). The MS conditions were as follows:
Ion source polarity: Positive ion mode
Sheath gas pressure (N2): 40 units
Ion transfer tube temperature: 350 °C
Collision gas pressure (Ar): 1.0 mTorr
Q1 resolution: 0.2 FWHM
Q3 resolution: 0.7 FWHM
Dwell time: 0.2 s
Scan type: SRM
Table 1 summarizes the SRM transitions that weremonitored. MS detection of the target compounds was dividedinto three time segments on the basis of their retention timesduring chromatography. The protonated molecular ion of thecompound [M 1 H]1 was selected as the precursor ion.Detection was performed in the multiple reaction monitoringmode using, usually, the two most intense and characteristicprecursor/product-ion transitions obtained from the MS–MSoptimization procedure. Identification criteria for the targetcompounds were based on the LC retention time (tR) and onthe ratio of the two monitored transitions for each compound.Method accuracy and precision were evaluated by recoverystudies, using deionized water spiked with appropriateamounts of the sulphonamides at three concentrations (2 ng/L,20 ng/L and 200 ng/L). Calibration plots were obtained byanalysis of standard solutions at eight concentrations in therange 0.1–100 μg/L (2–2000 pg injected).
Results and Discussion
The method validation data are summarized in Table 2.Linearity of the method was assumed because the r2 valueswere greater than 0.99 for the linear regression equations (1/xweighted) and the residuals were less than 20% for eachcalibration point in the concentration range 0.1–100 μg/L.Quantification was performed on the basis of externalcalibration plots using the peak area of the most intensetransition of the analyte. For SMX, quantification wasperformed using the ratios of the peak areas of the mostabundant monitored ion of the analyte to that of the respectiveion of the surrogate standard d4-SMX. The accuracy of themethod was determined by recovery studies conducted onspiked deionized water samples at three concentration levels:2 ng/L, 20 ng/L and 200 ng/L. The precision of the method wasdetermined by repeated intra-day (n 5 3) and inter-day analysis(n 5 6) of samples spiked at the three concentrations. Highrates of recovery were achieved, usually greater than 72% andrelative standard deviations for inter-day analysis (n 5 6)ranged between 3.1% and 19.0%. The SRM chromatogramsobtained from deionized water spiked at a concentration of2 ng/L are shown in Figure 2.
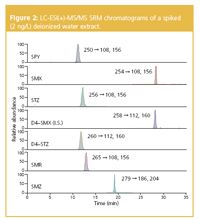
Figure 2
The method was applied to the wastewater samples toinvestigate the occurrence of sulphonamide antibiotics.Sulphamethoxazole was detected in all of the samples. Themedian concentration was 150 ng/L. The SRM chromatogramsof SMX in the wastewater effluent extract are shown in Figure 3.
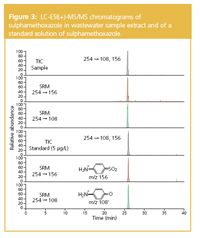
Figure 2
Conclusion
LC–ESI–MS–MS is a powerful analytical method for thesensitive determination of sulphonamide antibiotics inmunicipal wastewater at low ppt levels (ng/L). The solid-phaseextraction scheme for trace enrichment and separation ofsulphonamide compounds from wastewater samples yieldedhigh recovery rates and enabled their accurate quantification.Sulphamethoxazole (median concentration 150 ng/L) wasdetected in the wastewater effluent samples, which indicatedthat it was not completely eliminated in the sewage treatmentplants. The described method proved to be a valuable tool forthe detection of pharmaceuticals in wastewater effluentsbefore they reached the aquatic environment.
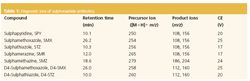
Table 1
References
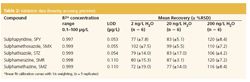
Table 2
1. M. Petrovic et al., J. Chrom. A, 1067(1–2), 1–14 (2005).
2. A. Göbel et al., Anal. Chem., 76(16), 4756–4764 (2004).3. S. Yang, K. Cha and K. Carlson, Rapid Commun. Mass Spectrom,18(18), 2131–2145 (2004).
4. J. Donn, M. Mendoza and J. Pritchard, “AP Probe Finds Drugs inDrinking Water.” Associated Press, 9 March, 2008,http://ap.google.com/article/ALeqM5hGsoyElv4ZL879LW6z2aZS0Pix7AD8VA14500.
5. P. A. Segura et al., J. Environ. Monit., 9, 307–313 (2007).
6. E. Botitsi, C. Frosyni and D. Tsipi, Anal Bioanal Chem., 387, 1317–1327(2007).
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



