Gradient Performance Tests for Reversed-Phase and Biochemical Applications using High Salt Buffers
In gradient mode, the quality of the analysis will be influenced by the performance of the proportioning and mixing of the eluents at any time during a given chromatogram.
To routinely obtain reliable results and develop HPLC methods efficiently, modern laboratories require not only advanced instruments and tools, but also precisely defined procedures for verifying performance. The reproducibility and accuracy of any HPLC analysis is strongly dependent on the solvent composition, especially when working in isocratic mode. In gradient mode, however, the quality of the analysis will be influenced by the performance of the proportioning and mixing of the eluents at any time during a given chromatogram. Therefore, routine testing of the gradient performance of the delivery system within well-defined intervals is, at least, of the same importance as testing column efficiency1,2 and selectivity.3,4
The most useful tests for ensuring the performance of a liquid chromatography system are the gradient linearity and step gradient tests. The necessity of performing such tests on a semi-annual basis has long been established.5 Simply testing the proportioning and dispensing of eluents by a given solvent delivery system when using water*/water, methanol*/methanol or acetonitrile*/acetonitrile gradients (*spiked with a tracer substance) may be sufficient. However, although most analytical applications requiring gradient elution use water or acetonitrile as eluents, most biochemical LC techniques such as ion exchange chromatography (IEC), hydrophobic interaction chromatography (HIC) or affinity chromatography use aqueous buffers containing up to 1 or 2 M salt concentrations. Particularly, for these types of applications, it becomes apparent that gradient performance is not only influenced by the proportioning of solvents but also by the efficiency of the mixing device itself. Therefore, it is advisable that a test procedure be used that is based on real chromatographic conditions (i.e., proportioning and mixing of aqueous/organic solvents as well as of aqueous buffers/concentrated salt buffers). Such tests should also enable additional information concerning linearity and stray light of the detectors used.
In this article we describe several powerful tests for gradient performance and accuracy using a mixer for inorganic and organic solvents.

Figure 1
Experimental Conditions
All measurements were performed using Knauer Smartline HPLC systems in both low pressure gradient (LPG) and binary high pressure gradient (HPG) modes. The HPLC system consisted of one or two pumps 1000, manager 5000 equipped with degasser and quaternary gradient unit, column oven 4050, UV detectors 2500 and 2600 equipped with 245 nL and 10 µL flow cells, respectively. The SmartMix static mixer equipped with either analytical or micro cartridge (depending on the flow-rate) was used for gradient mixing in both LPG and HPG systems. Salt gradients were monitored with the Monitor pH/C-900 from GE Healthcare equipped with a 10 µL flow cell. Varying lengths and inner diameters of 360 µm outer diameter fused silica capillaries were used as restrictors to achieve sufficient back pressure, ensuring good operation of the pump check valves and for testing gradient mixing.

Figure 2
Buffers were always freshly prepared and vacuum filtered through a 2 µm membrane prior to use. Moreover, eluents were always continuously degassed, either by on-line vacuum or by bubbling with helium.

Results
The suitability of the static mixer to efficiently mix polar aqueous solutions with organic hydrophobic solvents was evaluated. This mixer can be rotated (mounted upside down) to convert it from an LPG to an HPG static mixing unit. By using interchangeable cartridges, the mixing unit was customized to provide the lowest dead volume for either analytical HPLC (350 µL) or for micro LC (< 20 µL). Flow-rates for micro HPLC applications were established using the integrated flow splitting function.
To achieve micro flow-rates from an analytical pump, a restrictor (e.g., an appropriate length of fused silica capillary) is connected and the analytical cartridge is replaced with the micro cartridge, optimized for flow-rates from 1 to 500 µL/min.

Figure 3
Gradient performance tests Four major areas of application can be identified for gradient tests:
1. Test of solvent delivery system (malfunctioning check valves, leakage, etc.)
2. Test of gradient performance in typical analytical HPLC (low pressure gradient proportioning valve failure, electronic control of pumps, etc.)
3. Biochemical applications where the generation and mixing of high salt buffer gradients is required
4. Linearity and influence of stray light of detectors.
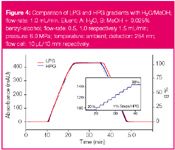
Figure 4
Step gradients at different flow-rates are not just good tests of mixing efficiency but of proper pump function and proper gradient proportioning valve function as well (Figure 1). Gradients with steps that are more than 10 minutes long are especially useful for checking the reliability of the eluent composition and for the development of isocratic analysis conditions for routine analysis. In addition, this type of gradient test also serves to check the linearity of the detector. It is recommended that benzyl alcohol (0.02% v/v) used as the UV-absorbing component in eluent B instead of acetone because the absorption coefficient of acetone is dependent on the composition of the eluent in which it is present.6 This is particularly important when running an aqueous eluent as eluent A and an organic eluent as eluent B. The mixing performance achieved with the static mixing device is shown in Figure 2. The same quality of gradient performance can also be easily achieved for narrow-bore HPLC when the delivery and proportioning systems are functioning properly, even when flow-rate is in the nanolitre scale. Capillary LC flow-rates can be established using the integrated flow splitter function.

Figure 5
For analytical scale work, which gradient mode will provide better results (either a binary HPG system consisting of two pumps and a mixer or an LPG system consisting of proportioning valves, one pump and a mixer) is mainly just a matter of personal belief and the instrumentation available (see Figure 4). The influence on gradient performance observed under ultra-high pressure (90–100 MPa) conditions was also examined (Figure 5). Additional experiments to assess the effect of high temperature (<100 °C) by controlling the temperature of the eluent (a sensor was mounted inside the column at the column inlet) showed that gradient accuracy is not dependent on temperature (Figure 6).
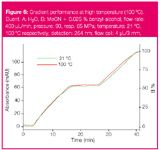
Figure 6
In the ever-expanding field of biochemical analysis many important applications such as amino acid analysis,7,8 enzymatic digests,9,10 sequence analysis11 and others are performed by reversed-phase (RP) HPLC. For these applications, gradients are usually generated by mixing aqueous eluents such as buffers with organic solvents, for example, acetonitrile or methanol. However, other chromatographic techniques, such as ion exchange chromatography (IEC), hydrophobic interaction chromatography (HIC) or affinity chromatography (AC), are of increasing importance12,13 for the separation of native biopolymers. For these applications, it is important to note that benzyl alcohol should not be used as a tracer in eluent B because it has very low solubility in aqueous salt solutions. Instead of benzyl alcohol, acetone, uracil or some other appropriate molecule for performance tests of such gradients could be used. Additionally, salt gradients offer the advantage of users being able to monitor the concentration of the tracer molecule by UV as well as the concentration of salt by conductivity. Simultaneous conductivity and UV detection in a step gradient enables users to check not only the performance of the gradient system itself but also to control the linearity of the detectors used (Figure 7).

Figure 7
Hydrophobic interaction chromatography, one of the most powerful tools for the isolation and purification of proteins, often demands a very high salt concentration when starting the separation (e.g., 2 M ammonium sulphate in eluent A). In this gradient test, the descending ammonium sulphate concentration can be easily monitored by conductivity while the programmed gradient is being tested. The ascending percentage of eluent B containing acetone as tracer is detected by UV at 265 nm (Figure 8). However, to retain the biological activity of proteins, the pH value must be simultaneously carefully controlled. In similar applications it may even become necessary to superimpose binary buffer salt gradients by a pH gradient, resulting in a ternary gradient requiring particularly efficient gradient mixing as enabled by the mixer described above. Reliable gradients are also necessary for sequence studies after the proteins are purified (Figure 9).
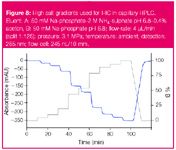
Figure 8
Conclusions
The static gradient mixer effectively mixes hydrophilic and hydrophobic eluents and can be used for both low pressure applications as well as high pressure gradient applications. Several modifications to typical gradient test procedures using water/water or organic solvent/organic solvent gradients were made for testing the additional performance required by modern biochemical applications that use high salt buffers. The routine controlling of gradient performance and accuracy guarantees high reproducibility and therefore enables reliable results, especially when developing new advanced HPLC methods.

Figure 9
Acknowledgements
N. Karima, University of Applied Sciences, Krefeld, Germany and Christian Schmidt, Wissenschaftliche Gerätebau Dr. Ing. Herbert Knauer GmbH, Berlin, Germany.
References
1. P.A. Bristow and J.H. Knox, Chromatographia, 10, 279–288 (1977).
2. V.R. Meyer, Practical High Performance Liquid Chromatography, third edition, (John Wiley & Sons, Chichester, England, 1999), pp. 120–135.
3. N. Tanaka et al., J. Chromat. Sci., 27, 721 ff. (1991).
4. H. Engelhardt, M. Arangio and T. Lobert, LCGC N. Am., 15(9), 856–866 (1997).
5. J.J. Gilroy and J.W. Dolan, LCGC Europe, 19(9), 454–458 (2006).
6. K. Shoikhet and H. Engelhardt, Chromatographia, 38(7/8), 421–430 (1994).
7. S.A. Cohen and D.P. Michaud, Anal. Biochem., 211, 279–287 (1993).
8. J. Naidanow et al., LaborPraxis, 6, 56–59 (2005).
9. E. Sterchi and W. Stöcker (Eds.), Proteolytic Enzymes: Tools and Targets, (Springer, Heidelberg, Germany , 1999).
10. C. Stuhlfelder, F. Lottspeich and M.J. Müller, European J. of Pharmaceutics and Biopharmaceutics, 53, 107–113 (2002).
11. F. Lottspeich and J.W. Engels (Eds.), Bioanalytik, (Spektrum Akademischer Verlag, Heidelberg, Germany, 2006), pp. 311–328.
12. R.K. Scopes, Ed., Protein Purification, (Springer, Heidelberg, Germany, 1994).
13. S. Hjertén et al., J. Chromatogr., 359, 99–109 (1986).
Silvia Marten,1 Anke Knöfel2 and Peter Földi,3
1Wissenschaftliche Gerätebau Dr. Ing. Herbert Knauer GmbH, Berlin, Germany,
2University of Applied Sciences, Berlin, Germany,
3University of Applied Sciences, Krefeld, Germany.
Silvia Marten has been head of Knauer's Applications and Columns department since 2004. She is a trained chemist specializing in instrumental analysis. Her research interests include investigation of the separation behaviour of HPLC phases, chiral stationary phases and miniaturization in HPLC.
Anke Knöfel is a student of biotechnology at the University of Applied Sciences, Berlin, Germany. Her diploma thesis focuses on the development of capillary HPLC columns and biochemical applications of capillary HPLC.
Peter Földi lectures in the Biotechnology Institute at the University of Applied Sciences Niederrhein, Krefeld, Germany. He has many years of experience in the fields of biochemistry and biotechnology and has been active in the enhancement of standard procedures and instrumentation for these fields in both the industrial and university research sectors.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





