Five Steps in the Evolution of an Instrumental Analysis Course for Enhanced Student Preparation
I have been at The University of Texas at Arlington for almost 10 years, and I have taught approximately seven different classes in my time here. Yet, the one that I have had the most opportunities to teach, and the most time to reform, is our junior/senior-level Instrumental Analysis (IA) course.
I have been at The University of Texas at Arlington for almost 10 years, and I have taught approximately seven different classes in my time here. Yet, the one that I have had the most opportunities to teach, and the most time to reform, is our junior/senior-level Instrumental Analysis (IA) course. This is the second course in our undergraduate chemistry-major curriculum; a prerequisite is the sophomore-level Quantitative Analysis course. Through the past 10 years, I have taught IA approximately 10 times, and I have made it a point to refine the course a little bit each time I teach it to improve student learning. My goal is to provide a course that gives our majors practical experience with tasks similar to those they might expect to receive when they move on in their professional careers. I regularly expound that no matter what area of chemistry or science these students ultimately pursue for their career, it is essential to have knowledge of analytical techniques to determine what or how much of something is in a sample.
IA is taught as a four-credit-hour course, where two credit hours are devoted to lecture (two hours of lecture are scheduled weekly through the semester) and two credit hours are devoted to laboratory work. The students are scheduled for two 4-h blocks of time in the laboratory each week. We currently serve approximately 20 students each semester in the course; we limit the laboratory portion to 8–12 students per section (two to three students per group). One graduate teaching assistant (TA) is allocated to each section, and these TAs are generally chosen from a pool of more seasoned, analytically oriented graduate students. Design of the laboratory experiments is largely dictated by space and the availability of instrumentation. We have approximately one or two of each type of instrument, with a little more variation in flavor when it comes to chromatography systems. Our strong university partnership with Shimadzu Scientific Instruments has significantly increased the availability and variety of instrumentation in the past couple of years - especially the augmentation of available instrumentation to include gas chromatography–mass spectrometry (GC–MS) and liquid chromatography (LC)–MS instruments for the students to use.
I have spoken to many other analytical chemistry educators, and I know that the IA course is one where they have placed quite a bit of thought and innovation for the benefit of optimally educating their students. In the original embodiment of the course here at U.T. Arlington, after students performed a large series of cookie-cutter experiments on six different instruments where the experimental procedure was laid out like a recipe and virtually guaranteed to work, they would be given a “practicum” that required them to design their own experiment and carry out the analysis. Given that students were used to cookie-cutter experiments in pretty much all of their other lab courses before IA, they were used to this configuration, but they enjoyed the ability to think outside the box at the end of the semester to complete their practicum. Even so, there was also frustration, because often the experiment did not work precisely as planned and some level of troubleshooting was necessary to arrive at a workable analysis and reasonable result.
That response made me think. As analytical chemists, we know that much time can be spent troubleshooting. In fact, the best analytical chemists are the ones who have experienced and can quickly diagnose problems. Collecting data in analytical experiments can be quite fast, but to ensure the data is of high quality, one must be aware of the pitfalls that can occur along the way. Problems with instruments can take time and patience to fix. I felt that an opportunity existed to significantly advance the real-world experience of the students in my IA course, so I have refined it to be more real-world in nature. Previously, I would estimate that the course was lacking relative to other innovative offerings at other institutions. Now, I believe that the students’ experience in my course is quite unique.
Here are five steps that I have taken to enhance student preparation through a continually modified IA course. Presented is the current model, which has been incrementally refined over time.
1. Problem-Based Learning
Analytical chemists are trained to proceed through a punch list of questions when posed with a new problem. This list includes questions about the nature of a sample, the analytes of interest, their levels, how much sample is available, the potential for interferences, and what type of method should be used, among others. Refining these questions, and their relative level of importance, as well as identifying potential pitfalls is essential to designing and executing an effective methodology to solve a problem - and this approach takes practice. It is for this reason that I transformed the IA lab into a problem-based learning environment.
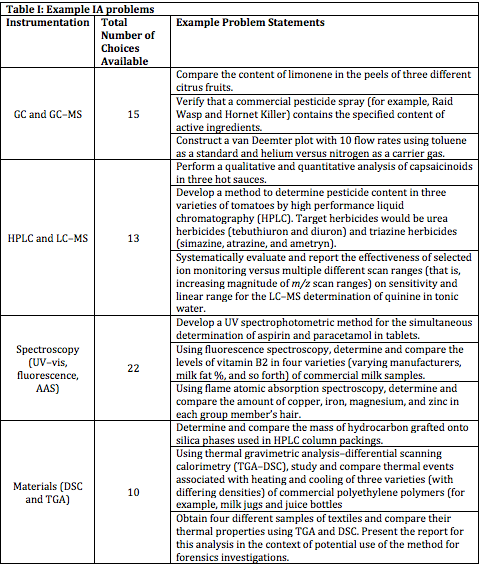
Generally speaking, there is enough time during the semester for students to perform four problem-based experiments. A list of problems has been generated and modified over time so that each student group, at the beginning of each rotation, can pick a problem in which they are interested. The problems are arranged in four main topics according to the type of instrumentation they will use: gas chromatography, liquid chromatography, spectroscopy, and materials analysis. The students rotate through the four areas throughout the semester, and they have at least three weeks (six lab periods or 24 total hours) to complete a pre-experiment (a very short cookie-cutter experiment to familiarize them with the instrumentation) and then to solve their chosen problem. For their problem, they are only given statements such as the examples listed in Table I. After one group in a semester chooses a problem, no other group in any section is allowed to repeat that problem - it is taken off of the list of available choices. In some cases, students may be allowed to pursue a problem that they themselves formulated (for example, “What is the actual amount of gold contained in the golden flakes present in a bottle of GoldSchläger liqueur?”). Students submit a requisition and procedures form to request the chemicals and consumables they need, and then they design and carry out the experiment. Reporting and assessment are addressed below.
2. Assessment of Laboratory Activities
Assessment of laboratory activities is crucial for a variety of reasons. In our case, we want to ensure that the students are prepared to address their problem, that they can design and execute a scientifically sound experiment, and that they can communicate their results in a variety of formats. To ensure that the students are prepared, we have three checks, each worth a portion of final grade (grading is discussed in more detail below). As mentioned, for each cycle, the students must first submit a requisition and procedures form. Based on this information, laboratory instructors can ensure that the students are on the right track. On average, we account for each group to be able to spend about $100 per experiment. In many cases, standards and reagents that have been purchased in past years are already available; supplies that are not at hand must be ordered as early as possible to avoid delay. The students also are given a quiz each cycle. For this quiz, they are given ahead of time a list of questions from which two will be selected for which they have to provide written answers. And then to round out the preparation, each student must submit a two-page pre-experiment report that details the results they obtained on their brief cookie-cutter introduction to each instrument.
After the problem-based experiment has been completed, the students must provide a comprehensive report of the background, experimental design, and results of their work. Given that there are four cycles, we have designed four separate formats for communication of that information and assessment. Their first laboratory report is to be formatted like a research article, using the Journal of the American Chemical Society (JACS) communication template. This template limits the students to three formatted pages and careful selection and presentation of their research results. In all reports, at least five references from the primary literature must be cited. Students prepare their JACS articles for submission to their TAs individually; thus, they can work together to design the experiment and solve the problem, but their reports must be individualized. All reports are graded by both the TA and the course instructor.
For their second cycle, the students are asked to report their experiments in the form of a standard operating procedure (SOP). Because many scientists may be required to prepare such documents in their professional career, this exercise gives them an experience that many in their undergraduate curriculum would not normally have. There is a variety of guidance on formatting an SOP on the web. In grading the SOP, we adjust the scoring rubric to represent the necessary presence of aspects that might not normally be communicated in a regular article (for example, detailed safety considerations, how to accurately prepare stock solutions, and so forth). Again, each student is required to submit his or her own SOP document for grading.
In the third cycle, the students in each group work together to create a poster presentation. While this is a common skill practiced by researchers, not all undergraduate majors participate in research (unfortunately). The students are given a generic template, but they are given artistic license to alter it as they desire to best communicate their work. The poster is submitted as a soft copy for grading, and each group must also give a 5-min presentation of their work to the rest of the lab section, and answer questions. The grading rubric includes scoring for the presentation part of the exercise.
Finally, in the fourth cycle, each group prepares an application note featuring their experiment. An application note is basically an abbreviated research article that emphasizes instrumentation settings and capabilities. The idea is that the final product can actually be submitted to the instrument manufacturer to be considered for publication and distribution. Because of this possibility, the submitted product undergoes some significant revision by the TAs and instructor. Not all application notes are of sufficient quality to submit to instrument manufacturers. Of course, a manufacturer is only going to publish a note that features high quality data. That said, if an application note is published, the student can then put it on his or her CV or résumé (even if it is not peer-reviewed). This would make for a very nice discussion point at a professional interview or in a job application. We have taken this route only once in the course so far, but out of eight submitted application notes, four were judged to be of sufficient quality to be prepared for submission to the instrument manufacturer. Again, because we have such a nice working relationship with Shimadzu, the process for pursuing such an interaction is streamlined - but that should not be a limitation.
The last component of the lab grade is a small amount of points awarded by the TA to each student for good laboratory practice. In this way, some part of the student’s grade is tied to attendance, participation, and behavior (cleanliness, being on-task, and so forth) in the laboratory.
3. Balancing Lecture and Laboratory Content and Curriculum
Because we have limited instrumentation in the lab, the groups must cycle through the different techniques. Thus, in one section, one group might start with gas chromatography in the first cycle, while another begins with a spectroscopy problem. Because the students take the lab concurrently with the lecture, it is difficult to provide appropriate background in each instrumental technique before the students embark on using it in the laboratory. This problem has caused me to reorganize the lecture content, so that important practical background information is given as soon as possible. Of course, students still have to take it upon themselves to read ahead a bit, especially to answer quiz questions in the lab, but some of the blindness can be mitigated.
I used to divide the course into four parts: a review of essential quantitative analysis topics (important things you forgot from your last analytical course), spectroscopy, chromatography, and mass spectrometry. This approach flows well in terms of lecture, but for chromatography, which is an area where students struggle the most in the lab, they were only receiving classroom instruction on the topic in the second half of the semester. Most complaints in end-of-course surveys were associated with difficulties performing HPLC experiments. To fix this, I condensed the quantitative analysis review into one to two lectures, and provided additional review material on-line. At the same time, I now ask students to complete introductions to theory and hardware for GC and HPLC on the ChromAcademy web site (www.chromacademy.com). This tutorial site is excellent. It is interactive, and it is free for use by students. In the classroom, I now take the first half of the course and cover fundamentals of the instrumental topics in a series of short lectures; a heavy emphasis is placed early on practical aspects of instrumental use that will help students in the lab, particularly in chromatography. I reserve the second half of the course to expand on these topics with more-detailed information. Whenever possible, I insert a couple of guest seminars that feature the use of the instrumentation in research endeavors.
4. Lecture Structure - Less Is More
As a starting faculty member, I was under the impression that it was essential to cover everything in the course. I now believe that it is better for the students to know a few essential techniques in depth, rather than knowing a little about everything. This stance allows me to be more selective in the amount of material I convey in standard lectures. My ideal classroom interaction opens with solicitation of any questions from the class. These are usually administrative in nature, but occasionally someone asks for clarification of a topic covered previously. I then give a 25–30 min presentation on a given topic. That is the plan, anyway. Sometimes various questions cause the discussion to deviate a bit from the plan, but that is fine. In the remainder of the course, I specifically solicit questions about the experiments they are performing in the laboratory.
Getting the students to speak up about questions they have on experimental design and instrument operation in the classroom can be challenging. Sometimes I simply have to ask a group what they are working on. Nevertheless, my goal in this exchange is to help the students make good decisions that will help them successfully solve their problem. Sometimes that is as simple as suggesting a calibration range for their experiment. Other times, it might be giving them some special considerations for handling interferences or choosing appropriate solvent systems. I like to view this in a similar manner to how I would interact with one of my students in my research group. I want to be there to help them get it right, rather than to leave them hanging out to dry because they didn’t think to put an acid modifier in their mobile phase, or something simple (and easily overlooked) like that. I still find that most groups would rather talk to me about their problems after class, and that is fine; the essential message to convey is that we are trying to build experience in this course. Part of that experience is learning from mistakes and troubleshooting; the other part is gauging what are the most important considerations for successfully solving a given problem.
5. Grading - It’s Necessary, But I Am Not Here to Fail Our Juniors and Seniors
It is my opinion that the only way a student can get a “D” or “F” in the IA course is to not come to class and to not turn in work products. I used to split the points that could be accumulated over the lecture and laboratory portions of the course evenly. I have changed that split recently by placing a much larger emphasis on the laboratory portion of the course. As I said, if this is to be a course where students gain the hands-on skills that they will take into the next step of their professional skills, I wanted the grading to emphasize that importance.
Thus, currently, out of 1000 points that can be earned, 200 points are earned through in class exams, and 800 points are earned in the laboratory assessments. The lecture points are earned on two in-class exams; although, I have changed these to take home variants in the past. Content tested in each is roughly split on concepts presented in the first half and the second half of the course. Half of the laboratory points are associated with the four final reports (JACS, SOP, poster, and application note; 100 points each). The remaining laboratory points are associated with the requisition form, the pre-experiment report, the quiz, and the good laboratory practice assessment. I emphasize to the students that the extra effort that they normally might put into studying notes in the course to prepare for exams should be placed on reading the literature, and understanding the application of the instruments in practical experiments. While it is difficult to enforce such an approach, for those students who do spend the extra time on their laboratory work, the effort shows.
I have no doubt that the course design that I have outlined above will continue to be modified. I take student course evaluations very seriously, and I am constantly soliciting feedback from the students and the TAs on how to make the course better. For that matter, I would be very happy to hear from LCGC readers about their experiences, or things they think might benefit the course or better prepare students for the work world. We have given some formal surveys to students in the past. What is clear is that at the outset of the course, students are very wary of the design of the laboratory. It is certainly easier to follow a written recipe and generate a report on an experiment where everything works. However, by the end of the semester, the students indicate that they enjoy and appreciate the problem-based nature of the course. They realize that this is precisely how they are going to be expected to operate in the real world. In many cases, I have had students who have gone off to graduate school or to work in industry. They have communicated their sincere appreciation for the preparation the IA course afforded them. In the end, that is the best reward for the effort.
Previous blog entries from Kevin Schug:
The LCGC Blog: Responsible Unconventional Oil and Gas Exploration in Colombia
The LCGC Blog: Intact Protein Separations: Some Education is Missing
The LCGC Blog: Evaluating the Impact of Unconventional Oil and Gas Extraction on Groundwater
The LCGC Blog: My New Obsession: Gas Chromatography with Vacuum Ultraviolet Absorption
The LCGC Blog: Unanticipated Benefits of Keyword Searching the Scientific Literature
The LCGC Blog: A Report from Riva del Garda: The Current State of the Art of Gas Chromatography
The LCGC Blog: Basics, Applications, and Innovations in Solid-Phase Extraction
The LCGC Blog: My Own March Madness
The LCGC Blog: A View of Separation Science Research at a Czech Conference
The LCGC Blog: What is the Optimal Training to Provide Students Interested in a Career in Industry?
The LCGC Blog: A Closer Look at Temperature Programming in Gas Chromatography
The LCGC Blog: Back to Basics: The Role of Thermodynamics in Chromatographic Separations
The LCGC Blog: The Dimensionality of Separations: Mass Spectrometry Is Separation Science
The LCGC Blog: What Can Analytical Chemists Do for Chemical Oceanographers, and Vice Versa?

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)