Enantiomeric Separation of Commonly Used Chiral Corn Pesticides Using SFC
Special Issues
The analysis of chiral pesticide is needed to improve risk assessment and establish, in some cases, the justification for production of single- or enriched-enantiomer products. Chiral pesticides are commonly used throughout the agriculture industry and their very chiral nature can lead to enantioselectivities in their mechanisms of action. These differences can also impact their efficacies, toxicities, degradation rates, and persistence in the environment.
This report illustrates good suitability of Regis' chiral columns for analysis of chiral pesticides (Figure 1). While chiral HPLC is a traditional, analytical technique that is performed for this analysis, we report good results using SFC (supercritical fluid chromatography) analysis. SFC is a technique that allows for fast and efficient separations of non-chiral and chiral compounds. It is known for its lower environmental impact, unlike its classical counterpart, liquid chromatography, and therefore could be advantageous.
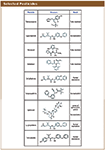
Experimental Conditions
SFC analysis was performed on the Waters Investigator SFC System. Regis chiral columns - RegisPack, RegisCell, and Whelk-O1 (25 cm × 4.6 mm, 5 µm) - were utilized for method development as the analytical columns.

Conclusion
Chiral separations of commonly used chiral corn pesticides using SFC are reported. Screening was done on chiral columns with various solvent conditions. A set of complementary chiral columns and method development was necessary to achieve selectivity for different compounds. While good results are reported for enantiomeric mixtures, results varied for mixtures of diastereomers and still require further improvement (Figure 2). Compared with traditional HPLC chiral methods, the SFC chiral analyses are usually more efficient. This means methods are faster with good selectivity making SFC the technique of choice.

Regis Technologies, Inc.
8210 Austin Ave., Morton Grove, IL 60053
tel. (847) 583-7661, fax (847) 967-1214
Website: www.registech.com/chromatography
Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



















