In Defense of Nitrogen as a Carrier for Capillary GC
There has been much written about the use of nitrogen as a carrier gas for capillary GC. Formerly, to say it wasn’t any good. Latterly to say that it’s pretty good and a better alternative to Helium than hydrogen from a practicality standpoint.
There has been much written about the use of nitrogen as a carrier gas for capillary GC. Formerly, to say it wasn’t any good. Latterly to say that it’s pretty good and a better alternative to Helium than hydrogen from a practicality standpoint.
Here, I present a simple case study to highlight how we used Nitrogen as a replacement for Helium. The point is, no matter what the Van Deemter plots for the various gases may tell us about the relative inherent efficiencies of the various carrier gases over a range of sensible linear velocities, it’s actually all about generating the efficiency which allows you to carry out your analysis and generate fit for purpose data.
You really only need to know a few straight forward facts in order to make Nitrogen a viable alternative, which you can obviously generate in your lab without having to rely on cylinders or helium, which becomes increasingly expensive.
Nitrogen isn’t particularly efficient at the linear velocities used with 0.25 and 0.32 mm i.d. columns in capillary GC (30–50 cm/sec.). It produces narrowest peaks at linear velocities around 10 cm/sec., the type of velocities that were produced by traditional packed GC columns, because they had a much higher internal diameter (4 mm typically).
Separations using Nitrogen on the capillary columns we typically use, will mean analysis times are 2 to 2.5 times longer, as we need to slow down the carrier flow in order to get narrow peaks.
But do we need peaks which are really narrow? Many separations that I see could lose a lot of plates before the resolution between peaks is compromised.
However, there is a way to claw back some of the "lost" efficiency, without having to have longer analyses.
If we look at a Van Deemter plot for columns of various different internal diameter we will see that the optimum linear velocity (regardless of which gas is involved), moves to a higher value (Figure 1).
Changing from Helium and Nitrogen While Maintaining Separation Efficiency and Analysis TimeThe Column Oct 26, 2015 Volume 11, Issue 19
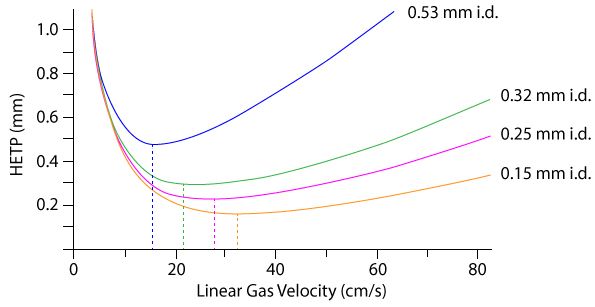
Well, if we reduce the internal diameter of a GC column, we can "win back" some of the efficiency reduction we see when using Nitrogen. If we adjust the flow rate down to around 10 cm/sec., reducing the column i.d. would mean even longer retention times!So – what does that mean?
However, we can increase the linear velocity of the carrier and perhaps even shorten the column, to ensure that overall analysis time is approximately similar. If the column is shortened and the i.d. narrowed, you can preserve the approximate analysis time by adjusting the carrier flow (linear velocity) so that the holdup time is matched to that of the original method.
Further, by matching as closely as possible, the phase ratio (b), which is the ratio of the volume to carrier to stationary phase within the column, we can preserve the selectivity of the separation.
So simply put;
- Use nitrogen carrier
- Reduce the column i.d. to use narrow columns (0.18mm i.d. columns can be used without making other adjustments to the instrument)
- Shorten the column
- Preserve the phase ratio
This is difficult to do in your head, so you might want to use one of the many GC method translators out there. We did this and here are the results with a few explanatory notes;
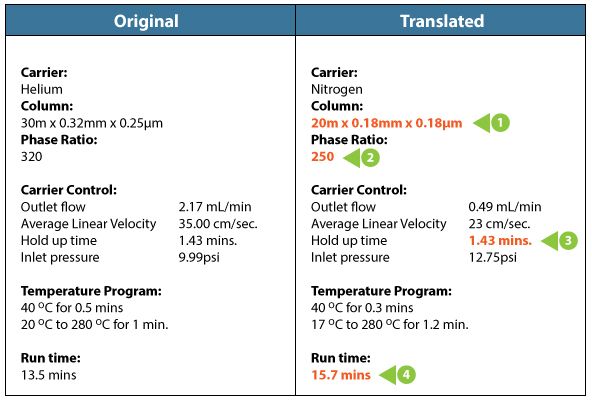
Table 1: Method conversion parameters for the original and new methods.
So we tried the methods on both columns, and Figure 2 shows expanded regions of the trickiest part of the chromatogram to see what the slight reduction in efficiency and the slightly altered phase ratio meant for the method.
Figure 2: Section showing the most critical peak pairs for the original and converted methods for a pesticide formulation.
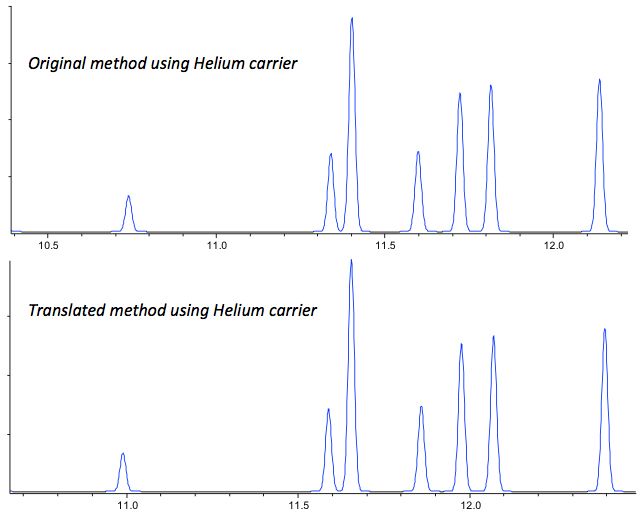
I’m sure you agree that the original and converted methods look very similar. The figures of merit including resolution, efficiency and symmetry all also check out very well.
I know you aren’t all going to rush into the lab and start to reconfigure your GC’s, but seriously, having a gas which can be generated in the laboratory makes a lot of sense both in terms of convenience and cost. Doing the ROI calculation of capital investment in a Nitrogen generator against the long term use of Helium gives you a pretty short break-even timeframe.
Nitrogen is a perfectly acceptable alternative carrier gas for many capillary GC methods with some adjustments to the column geometry to mitigate any loses in efficiency.
For more information, contact either Bev (bev@crawfordscientific.com) or Colin (colin@crawfordscientific.com). For more tutorials on LC, GC, or MS, or to try a free LC or GC troubleshooting tool, please visit www.chromacademy.com.
.
The LCGC Blog: Historical (Analytical) Chemistry Landmarks
November 1st 2024The American Chemical Society’s National Historic Chemical Landmarks program highlights sites and people that are important to the field of chemistry. How are analytical chemistry and separation science recognized within this program?
