Comparison of a UPLC Method across Multiple UHPLC Systems
In 2004, Waters introduced the ACQUITY UPLC® System. Since this launch, many liquid chromatography (LC) vendors have introduced modified high-performance liquid chromatography (HPLC) systems designed for ultra-high-pressure liquid chromatography (UHPLC). Although these systems may provide satisfactory performance for analytical-scale compressed chromatography (4.6-mm I.D.), they struggle significantly to provide high-resolution chromatography with sub-2-μm microbore columns (2.1-mm I.D.), which require a system designed to maximize the separation efficiency.
Tanya Jenkins, Waters Corporation, Milford, MA, U.S.
INTRODUCTION
In 2004, Waters introduced the ACQUITY UPLC® System. Since this launch, many liquid chromatography (LC) vendors have introduced modified high-performance liquid chromatography (HPLC) systems designed for ultra-high-pressure liquid chromatography (UHPLC). Although these systems may provide satisfactory performance for analytical-scale compressed chromatography (4.6-mm I.D.), they struggle significantly to provide high-resolution chromatography with sub-2-μm microbore columns (2.1-mm I.D.), which require a system designed to maximize the separation efficiency.
Typically, vendors of modified HPLC systems will claim improvements in sample throughput and a reduction in solvent consumption by migrating traditional HPLC methods to analytical-scale UHPLC methods, rather than discuss resolution. However, the transition to an analytical-scale UHPLC method yields only a small percentage of solvent savings compared to converting the method to a microbore-UPLC® method. Solvent consumption can be further reduced by nearly 5X or 80% with a 2.1-mm I.D. column compared to a 4.6-mm I.D. column of the same length.
Additionally, the improvements in the separation quality generated by a low-dispersion UPLC System provides the user with higher quality information than that possible with HPLC systems modified for UHPLC. The ACQUITY UPLC System is the world's only system that is optimized out-of-the-box to deliver high-resolution, low-volume liquid chromatography.
This application note compares the performance of multiple vendors' UHPLC systems for the separation of a series of anesthetics using an ACQUITY UPLC sub-2-μm column. It demonstrates that the performance of a modified HPLC system does not equal that of a holistically-designed UPLC System for achieving the highest separation efficiency, best sensitivity, and fastest analysis time.
EXPERIMENTAL
The method used to compare the four LC systems is as follows:

The same column, lot of mobile phase, and wash solvents were used on all the systems. Instruments were configured according to the manufacturer's recommendations for low system delay volume and, when possible, the shortest piece of 0.0025 in. I.D. tubing was used before and after the column to minimize peak dispersion. Depending upon the system, this included installing a microbore flow cell, reduced-volume mixers, reduced-volume tubing, bypassing pump components, and utilizing the bypass mode in the injector to further reduce gradient delay.
As a baseline for this comparison, Figure 1 shows the separation of the anesthetic mix on the ACQUITY UPLC System. No system modifications were necessary for the ACQUITY UPLC System since the stock configuration is optimized for high-resolution, low-dispersion UPLC analyses.
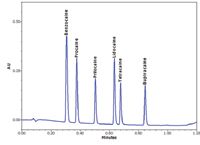
Figure 1. Separation of six anesthetics by UPLC on the ACQUITY UPLC System.
RESULTS AND DISCUSSION
For all the separation parameters assessed, the ACQUITY UPLC System, which was designed for high sensitivity and minimal band spread, easily outperformed all of the UHPLC systems.

Figure 2. Comparison of separation of the anesthetic mixture using four different vendors UHPLC Systems. The y-axis is fixed to demonstrate the impact of system dispersion and detector path length education on peak height.
Figure 2 compares the separation on each of the UHPLC systems with a fixed y-axis. Note that all of the other systems in the comparison had experienced reduced sensitivity compared to the ACQUITY UPLC System. This is a result of the shorter path length of the microbore flow cells used to reduce the extra-column band spread. It is evident that these systems were not designed to be compatible with high-resolution, low-volume separations, but rather modified in an attempt to compete with the ACQUITY UPLC System. If the y-axis is normalized, as shown in Figure 3, the effect of the increased system dispersion and the higher gradient delay is highlighted. The separation on the ACQUITY UPLC System has narrower peak widths than those on all of the other systems. Additionally, the early eluting peaks in the chromatograms have significantly greater peak widths than the later eluting peaks on most of the other systems, demonstrating the impact of both extra-column band spread from the injector, and the increased gradient delay volumes.
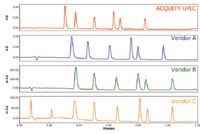
Figure 3. Comparison of the anesthetic separation on four different UHPLC systems. The y-axis is normalized to demonstrate the impact of the system volume and system band spread on the peak shape.
A summary of the critical separation and peak parameters that were assessed is shown in Table 1. The peak capacity of a separation is defined as the number of peaks that can be resolved during the gradient time. This is typically reported at 4.4% of the peak height (5 Sigma), which is indicative of resolved peaks. Some LC/MS literature will calculate peak capacity at ½ peak height. While it does not practically indicate the resolving power of a LC/UV separation, it has also been included for comparison. The peak width ratio compares the width of the most polar and least polar components in the separation. If the system dispersion and gradient delays are minimal, these values should approach 1, indicating an efficient gradient separation. The elution time of the last peak is also an indication of the system volume, as it requires the strongest part of the gradient to elute it off the column, and will define the final run time. When these values are plotted graphically (Figures 4 to 6), the performance benefits of the ACQUITY UPLC System are obvious. From these values, an assessment of the performance of each of the UHPLC systems compared to the ACQUITY UPLC System can be made.

Table 1. Comparison of critical separation parameters impacted by system volume and band spread.
The UHPLC system from Vendor A required hardware changes to the pump and detector, as well as an injection loop bypass function in the instrument method in order to reduce system volume. Even with these significant changes, the impact of high system volume on the gradient delay is apparent. The peak capacity for this separation using Vendor A's UHPLC system was 28% lower than the ACQUITY UPLC System. The lower peak capacity results in significantly reduced chromatographic resolution, which impacts the quality of information available to the user. Another indication that the system dispersion was too high for quality chromatographic results is the peak width ratio of the first and last peaks. This value indicates there is dispersion in the more polar components that typically results from the initial isocratic hold caused by the increased gradient delay, and/or extra-column band spread from the injector or column pre-heating assembly. The larger system volume and increased gradient delay resulted in longer elution times for each of the components compared to the ACQUITY UPLC System, and therefore required longer chromatographic run times. Additionally, a higher system volume will require longer system re-equilibration times resulting in even longer injection-to-injection cycle times.
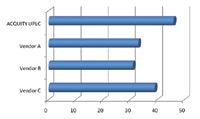
Figure 4. Comparison of peak capacity at 4.4% peak height highlights the increased resolving power of the ACQUITY UPLC System.
The UHPLC system from Vendor B did not require any hardware changes according to the system literature. The resulting separation had a peak capacity of 31, which was 33% lower than the ACQUITY UPLC System. The peak width ratio for the system from Vendor B appears to be the best of the three UHPLC vendors. However, when combined with the lowest peak capacity, this indicates that the first and last peak have very similar and high dispersion characteristics. This implies that the greatest contribution to extra-column band spread in this system is post-column, likely in the flow cell and its internal connection tubing. The elution time of the last peak indicates that the gradient delay on this UHPLC system was longer than the ACQUITY UPLC System and therefore the system volume is significantly higher.
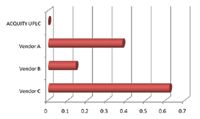
Figure 5. Comparison of the first-to-last peak width ratio deviation from 1 (ideal) demonstrates the impact of band spread on early eluting peaks.
The system from Vendor C required hardware changes to the pump, autosampler, and detector, as well as the bypass function in the instrument method to reduce gradient delay. Even with all these system changes, the resulting separation had a peak capacity that was 15% lower than that observed on the ACQUITY UPLC System. The first-to-last peak width ratio indicated that there was significant dispersion in the system that was the result of either the isocratic hold caused by the gradient delay, or from the injector/pre-heater assembly. The late elution time of the last peak indicates contributions to the run time from the gradient delay even though significant system modifications and the injection bypass mode had been implemented to reduce the system volume. The injector bypass function added a system peak at 0.22 min, which could easily be mistaken as an unknown peak in the sample.
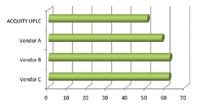
Figure 6. Comparison of the elution times of the last peak in the separation demonstrates the impact of system volume on the chromatographic run time.
CONCLUSION
This application note demonstrates the importance of a holistically-designed system for UPLC analysis. Although a sub-2-μm particle column provides high-resolution separations, a low-dispersion system is required to maximize the benefits of its resolving power. The design differences of LC systems can significantly impact resolution, sensitivity, sample throughput, and can ultimately impact the quality of the results generated in the laboratory.
For this UHPLC separation, the ACQUITY UPLC System easily outperformed all of the UHPLC vendors' systems with the greatest peak capacity, highest sensitivity, and fastest analysis time.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).





