Comparison of a Fast HPLC Method across Multiple LC Systems
Since the introduction of the Waters® ACQUITY UPLC® System, many vendors have introduced modified high-performance liquid chromatography (HPLC) systems designed for fast LC or ultra-high-pressure liquid chromatography (UHPLC). These systems, which can yield satisfactory chromatography at an analytical scale (4.6-mm I.D.), where system volume and system bandspread have less of an impact on peak width, struggle significantly with microbore chromatography (2.1-mm I.D.). These low-volume separations require a system designed to maximize the separation efficiency to provide greater quality information for the user.
Tanya Jenkins, Waters Corporation, Milford, MA, U.S.
INTRODUCTION
Since the introduction of the Waters® ACQUITY UPLC® System, many vendors have introduced modified high-performance liquid chromatography (HPLC) systems designed for fast LC or ultra-high-pressure liquid chromatography (UHPLC). These systems, which can yield satisfactory chromatography at an analytical scale (4.6-mm I.D.), where system volume and system bandspread have less of an impact on peak width, struggle significantly with microbore chromatography (2.1-mm I.D.). These low-volume separations require a system designed to maximize the separation efficiency to provide greater quality information for the user.
Liquid chromatography system vendors will claim improvements in resolution and sample throughput by migrating traditional HPLC methods to analytical-scale fast LC. Migrating a method from HPLC to fast LC is an attractive solution for businesses looking for ways to reduce the cost of analysis per sample and increase profitability; however the transition to an analytical-scale fast LC method only yields a small percentage of the solvent savings compared to converting the method to a microbore-scale fast LC method. Solvent consumption can be further reduced by nearly five times or 80% with a 2.1-mm I.D. column, compared to a 4.6-mm I.D. column of the same length, resulting in a significantly greater cost reduction per sample. In addition to the ACQUITY UPLC System being the world's only UltraPerformance LC® system, it is also ideally suited for fast LC or compressed chromatography (separation beyond the optimal linear velocity to maximize speed at reduced resolution), since it is optimized out-of-the-box for low dispersion chromatography.
This application note compares the performance of multiple LC systems for separation of a series of anesthetics on a microbore Intelligent Speed™ (IS™) Column. Although significant benefits can be realized by fast LC on the ACQUITY UPLC System, the greatest benefits are achieved by UPLC® separations. A comparison of multiple vendors for UHPLC performance is discussed in Waters Application Note, Comparison of a UPLC Method across Multiple UHPLC Systems, no. 720003166EN.
EXPERIMENTAL
The method used to compare the six LC systems was as follows:

The same column, mobile-phase lot, and wash solvents were used on all the systems. Instruments were configured according to the manufacturer's recommendations for low system delay volume. When possible, the shortest piece of 0.0025 in. I.D. tubing was used before and after the column. Depending upon the system, this included installing a microbore flowcell, reduced volume mixers, reduced volume tubing, bypass of pump components, and utilizing the bypass mode in the injector to further reduce gradient delay. Figure 1 shows the separation of the anesthetic mix on the ACQUITY UPLC System. No system modifications were necessary for the ACQUITY UPLC System since the stock configuration is optimized for ultra-low dispersion for both UPLC and HPLC applications, whether analytical or microbore scale.
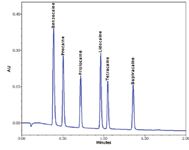
Figure 1. Separation of six anesthetics by fast LC on the ACQUITY UPLC System.
RESULTS AND DISCUSSION
For all the separation parameters assessed, the ACQUITY UPLC System, which was designed for high sensitivity and minimal band spread, easily outperformed all of the other LC systems for fast microbore LC. Figure 2 compares the separation for each of the LC systems with a fixed y-axis. Note that all of the other systems in the comparison had reduced sensitivity compared to the ACQUITY UPLC System. This was a result of the shorter pathlength of the microbore flow cells used to reduce the extra column band spread, and additional system dispersion. The other vendors' systems were not designed to be compatible with high-resolution, low-volume separations. If the y-axis is normalized, as shown in Figure 3, the effect of the increased system dispersion and the higher gradient delay is dramatically highlighted. The separation on the ACQUITY UPLC System had significantly narrower peak widths than all of the other systems. Additionally, the early eluting peaks in the chromatograms had significantly greater peak widths than the later eluting peaks on most of the other systems. This demonstrates the impact of both extra-column band spread from the injector and increased gradient delay volumes.
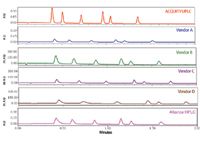
Figure 2. Comparison of the anesthetics separation of six different vendors LC systems. The y-axis is fixed to demonstrate the impact of system dispersion and detector path length reduction of peak height.
A summary of the critical separation and peak parameters that were assessed is shown in Table 1. The peak capacity of a separation is defined as the number of peaks that can be resolved during the gradient time. This is typically reported at 4.4% of the peak height (5 Sigma), which is indicative of resolved peaks. Some LC/MS literature will calculate peak capacity at ½ peak height (which does not indicate the resolving power of a LC/UV separation); therefore, this value has also been included for comparison. The peak width ratio compares the width of the most polar and least polar components in the separation. If the system dispersion and gradient delay are minimal, these values should approach 1, indicating an efficient gradient separation.
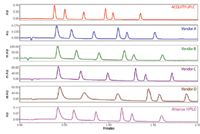
Figure 3. Comparison of the anesthetic separation on the six different LC systems. The y-axis is normalized to demonstrate the impact of the system volume and system band spread of the peak shape.
The elution time of the last peak is also an indication of the system volume as it requires the strongest part of the gradient to elute it off the column, and will define the final run time. When these values are plotted graphically, as shown in Figures 4 to 6, the performance benefits of the ACQUITY UPLC System (which was designed for high-resolution, low-volume separations) is dramatically realized. From these values, an assessment of the performance of each of the LC systems compared to the ACQUITY UPLC System can be made.

Table 1. Comparison of critical separation parameters impacted by system volume and band spread.
The system from Vendor A required hardware changes to the pump and detector, as well as an injection loop bypass function in the instrument method to reduce the system volume. Even with these changes, the impact of the gradient delay volume was apparent. The peak capacity was 34% lower than the ACQUITY UPLC System, resulting in significantly less resolving power. The peak width ratio of the first and last peaks indicated dispersion in the more polar components, which typically results from the initial isocratic hold imparted by the increased gradient delay and extra-column band spread from the injector or column pre-heating assembly. The increased gradient delay also caused longer elution times and therefore required a longer chromatographic run time. Additionally, Vendor A's higher system volume required longer re-equilibration times, resulting in even longer injection-to-injection cycle times.
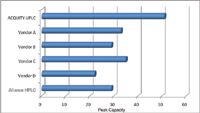
Figure 4. Comparison of peak capacity at 4.4% peak height highlights the increased resolving power of the ACQUITY UPLC System.
The system from Vendor B did not require any hardware changes according to its system literature. The resulting separation had a peak capacity of 29, which was 43% lower than the ACQUITY UPLC System. Despite the significantly lower peak capacity, the peak width ratio indicated that the first and last peaks had very similar dispersion characteristics, with a ratio near 1. This would indicate that the greatest contribution to extra-column band spread in this system is post-column, likely in the flow cell and its internal connection tubing. Additionally, the elution time of the last peak indicates that the gradient delay of this system is higher than that of the ACQUITY UPLC System.
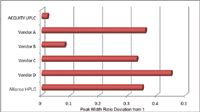
Figure 5. Comparison of the first-to-last peak width ratio deviation from 1 (ideal) demonstrates the impact of band spread on early eluting peaks.
The system from Vendor C required hardware changes to the pump, autosampler, and detector, as well as a bypass function in the instrument method to reduce gradient delay. Even with all these system changes, the resulting separation had a peak capacity that was 31% lower than the ACQUITY UPLC System. The peak width ratio indicated that there was dispersion in the system that resulted from either the isocratic hold imparted by the gradient delay, or from the injector/pre-heating assembly. Also, the late elution time of the last peak indicates contributions to the run time from the gradient delay, even though significant system modifications and bypass mode had been implemented.
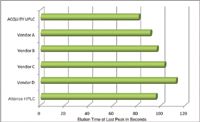
Figure 6. Comparison of the elution times of the last peak in the separation demonstrates the impact of system volume on the chromatographic run time.
The system from Vendor D recommended only a microbore flow cell and a low-volume tube from the injector to the column to reduce system dispersion. There were no options available for the reduction of the gradient delay volume (either hardware or software). This system had the lowest overall performance for this comparison. The peak capacity was 57% lower than the ACQUITY UPLC System. The peak width ratio was the highest of all the systems compared, and the elution time of the last peak was 38% longer than the ACQUITY UPLC System. Although this system had some components (microbore flow cell and low-volume tube) available to make it more compatible with fast microbore LC, it was definitely not intended to be used in this capacity.
As a point of comparison, the Waters Alliance® HPLC System was also included. To make the system compatible with fast microbore LC, the microbore flow cell (Part no. 205000400) was installed. The system pre-column volume setting in the instrument method was configured for 650 μL to reduce the gradient delay volume. The resulting peak capacity was in line with the values that were achieved with the other vendors' systems that were designed for fast LC and UHPLC. Additionally, the peak width ratios and the elution times achieved with the other vendors' systems were in line with those of the Alliance HPLC System, not those of the ACQUITY UPLC System, indicating that these systems truly perform within the realm of HPLC rather than that of UPLC.
CONCLUSION
For this fast LC method, the ACQUITY UPLC System delivered a separation that had the greatest peak capacity, highest sensitivity, and fastest analysis time.
This application note demonstrates the impact of LC design differences upon the overall quality of the LC separation, and the subsequent quality of the results generated by the laboratory. Careful consideration should be applied to the selection criteria of new LC systems to ensure laboratory workflow is not compromised by the perceived "need for speed." Today's ideal LC platform has an intrinsically low dispersion volume and other fluidic design considerations that allow it to reliably and accurately run conventional LC separations, fast LC separations, and sub-2-μm LC.
The 2004 introduction of the Waters ACQUITY UPLC System had these objectives in mind, and represents the best choice for laboratories that want to leverage UPLC's unique attributes to improve their workflow, and positively impact the bottom line of their businesses.
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.





