Assessing the Degradation of Ozenoxacin with Liquid Chromatography
In a recent study, Raja Sundararajan and Ashwinkumar Matta, scientists from the GITAM School of Pharmacy in Andhra Pradesh, India, used different liquid chromatography techniques to assess the properties of ozenoxacin (OXC) and its degradation products (1). Their work was published in Rapid Communications in Mass Spectrometry.
3D image of Ozenoxacin skeletal formula - molecular chemical structure of Impetigo medication isolated on white background | Image Credit: © kseniyaomega - stock.adobe.com
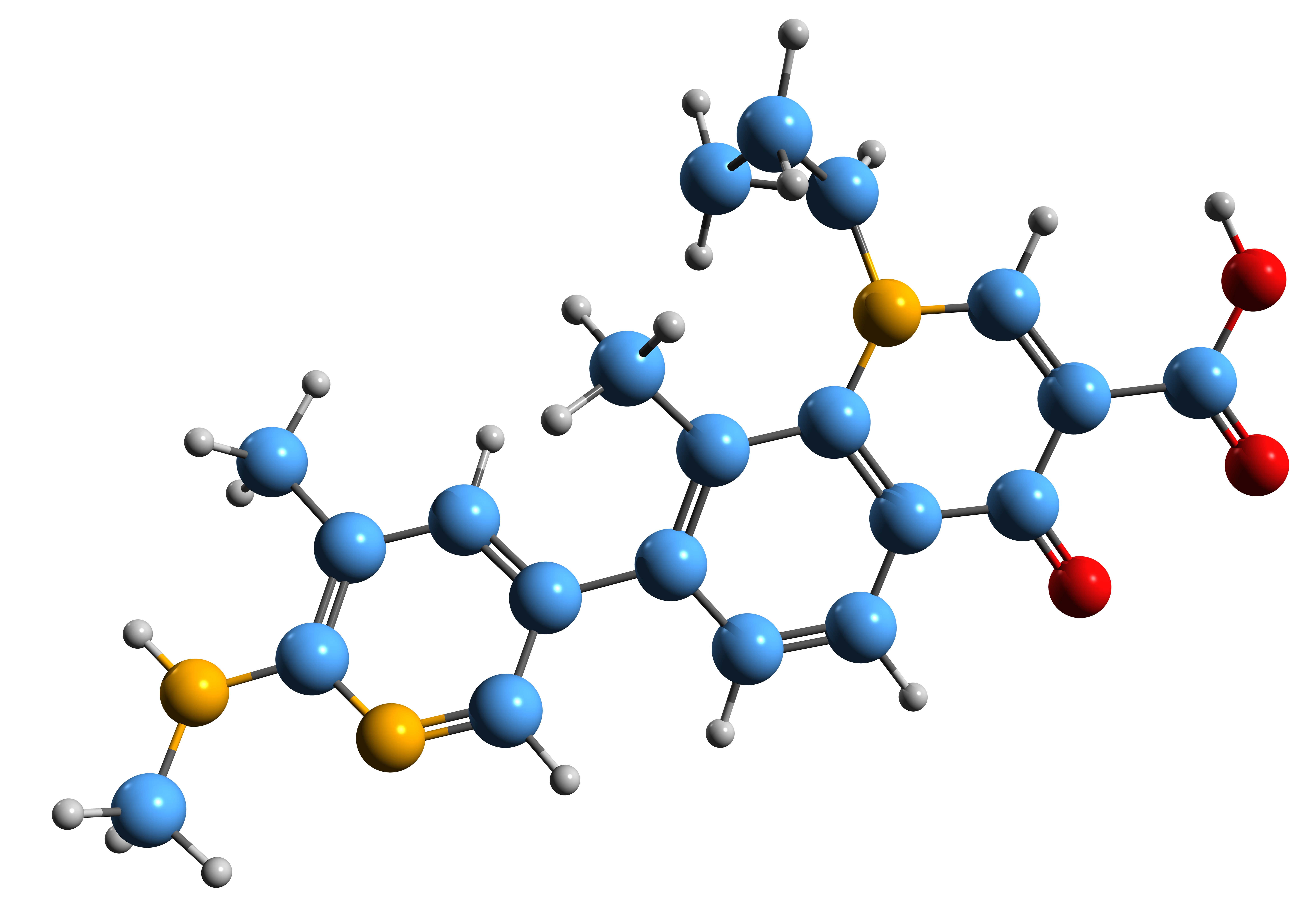
Impetigo is a highly contagious skin infection that mainly impacts infants and young children. Impetigo is caused by two types of bacteria, group A Streptococcus and Staphylococcus aureu, and it usually appears as reddish sores on the face, usually around the nose, mouth, hands, and feet (2,3). These sores eventually burst and develop honey-colored crusts. The condition is mainly treated by oral or topical antibiotics; one topical antibiotic used to treat impetigo is ozenoxacin, the subject of this experiment. For this study, the scientists wanted to evaluate the degradation products (DP) of OXC drug substances under different stress conditions. All experiments were in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines Q1A(R2) and Q1B.
Read More: Unlocking Metabolic Mysteries: Glioma Tumor Spheroids' Biochemical Blueprint Revealed
For this experiment, the OXC drug substances underwent forced degradation conditions, including thermal, oxidative, photolytic, and hydrolytic (neutral, acidic, and basic) degradation. Following these experiments, four DPs were formed under oxidative stress conditions with azobisisobutyronitrile (AIBN). The formed DPs were identified and separated using a Shimadzu LC system with a reversed-phase Phenomenex Kinetex C18 column, using 10 mM NH4CH3COOH buffer (pH−5.0) as mobile phase A and acetonitrile as mobile phase B, all with a detection wavelength of 254 nm (1).
Read More: Advancements in Tissue Lipidomics Hold Promise for Understanding Cardiovascular Health
The drug was found to be stable in neutral, acidic, basic, and oxidative degradation conditions with hydrogen peroxide. Liquid chromatography-electrospray ionisation-quadrupole time-of-flight-tandem mass spectrometry was employed in a positive ionization mode, with the aim being to analyze the drug and mass of the identified DPs. The mechanism and the pathway of mass fragmentation have been proposed, with the developed method being seen as accurate, repeatable, linear, and selective for further research. Predictor software was later applied to predict in silico toxicity for the drugs and their DPs, in addition to their physicochemical characteristics.
References
(1) Matta, A.; Sundararajan, R. Identification, Characterisation and In Silico ADMET Prediction of Ozenoxacin and Its Degradation Products Using High-Performance Liquid Chromatography-Photodiode Array and Liquid Chromatography-Quadrupole Time-of-Flight-Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2023, 38 (3), e976. DOI: https://doi.org/10.1002/rcm.9676
(2) Impetigo. Mayo Foundation for Medical Education and Research (MFMER) 2024. https://www.mayoclinic.org/diseases-conditions/impetigo/symptoms-causes/syc-20352352 (accessed 2024-3-20)
(3) Impetigo: All You Need to Know. U.S. Department of Health & Human Services 2022. https://www.cdc.gov/groupastrep/diseases-public/impetigo.html (accessed 2024-3-20)
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.






