Allowable Changes to Chromatography Methods for HPLC – More Freedom for Chromatographers Granted by the Pharmacopeias?
On December 1 2022, the newly harmonized General Chapter <621> (Chromatography) of the United States Pharmacopeia (USP) became official. The British Pharmacopoeia (BP), European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP) followed suit in January 2023.
Most chromatographers that use pharmacopeial methods will be aware of this chapter, and the various updates to the “System Suitability” subsection titled, “Adjustment of Chromatographic Conditions,” which have been released since 2009. Initially introduced to allow adjustments to chromatography conditions to meet system suitability requirements, this section has evolved to allow more widespread changes. These allowable changes can also be used to modernize monograph methods which use older HPLC columns to shorten analysis time, improve method performance, and reduce solvent usage. This is highly attractive not only to meet analytical throughput requirements, but to reduce the environmental impact of laboratory methods, which is becoming increasingly important.
I’ve previously written on various updates to this chapter (1,2) and given illustrative examples of how allowable changes may be implemented, but in this case, I want to focus on a significant change in the allowable changes to the column dimension and particle size changes which are now allowed with gradient chromatography methods, introduced in the latest update to the chapter in December 2022.
Before we get to this discussion, I want to draw attention to some important considerations for any chromatographer adjusting pharmacopeial methods.
The Adjustment of Chromatographic Conditions section outlines various allowable changes to Monograph methods and clearly states that changes outside of these ranges require full method revalidation. It is important to highlight that the section also states:
- Multiple adjustments can have a cumulative effect on the performance of the system and are to be properly evaluated by the users.
- If adjustments are made to a pharmacopeial procedure, additional verification tests may be required.
- Compliance with the system suitability criteria is required to verify that conditions for satisfactory performance of the test or assay are achieved.
- Adjustment of conditions with gradient elution HPLC is more critical than with isocratic HPLC, since it may shift some peaks to a different step of the gradient, potentially causing partial or complete coelution of adjacent peaks or peak inversion, and, thus leading to the incorrect assignment of peaks and to the masking of peaks or a shift such that elution occurs beyond the prescribed elution time.
These caveats are very important, especially where we are making several changes to a monograph method to take advantage of more modern HPLC technologies such as smaller particles, superficially porous particles (SPPs) and the attendant reduced column dimensions. In fairness, there will be few chromatographers working with pharmacopeial methods who would not undertake verification of the adjusted method, even if full revalidation is not mandated. Parameters which may be affected, and therefore need verification within the adjusted method, will depend on the nature of changes made, the purpose of the procedure (that is, assay or stability indicating method), and the ability of the system suitability requirements to verify that affected method characteristics remain valid. For assistance in deciding which criteria may be affected, one might refer to General Chapter <1226> Verification of Compendial Procedures. As stated in the caveats above, this is most important where adjustments to gradient methods are made because of the complexity of the retention mechanism. It is also important to remember that for gradient HPLC methods, further system suitability requirements are specified within the chapter, but more on that later. Lastly, it’s also important to remember that the allowable changes are all relative to the original monograph and not the version of the method in your own standard operating procedures (SOPs). Your in-house methods may have already had changes made against the original monograph and any further changes to these methods may take your overall adjustments beyond those of the allowable changes.
Table I below shows a summary of the allowable changes to monograph methods as defined within USP General Chapter <621>.
Table I: A summary of the allowable changes to monograph methods as defined within USP General Chapter <621>.
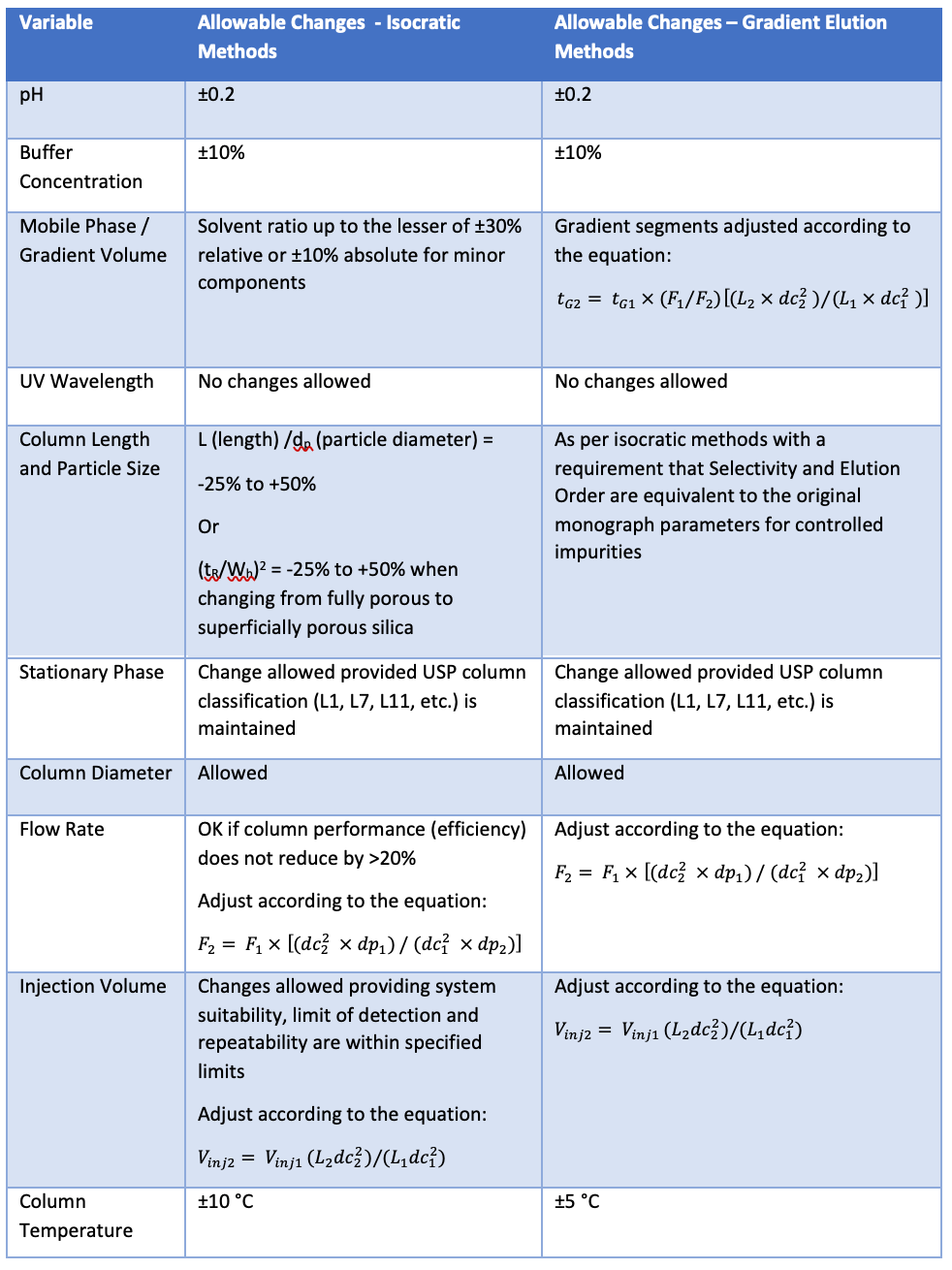
The most significant change in the latest update to General Chapter <621> is the possibility to alter the L (column length)/dp (particle diameter) ratio for gradient elution separations. As such, it’s worthwhile demonstrating how one might go about calculating the changes for a gradient elution HPLC monograph method, and the determination of organic impurities in Olanzapine formulations is a convenient example to use.
The monograph method for olanzapine impurity determination includes the following details:
Buffer: 68 mMol Sodium Dodecyl Sulphate (pH 2.5)
Solution A: Acetonitrile:Buffer(48:52)
Solution B: Acetonitrile:Buffer (70:30)
Mobile phase gradient: (see Table II below)
Table II: The mobile-phase gradient for solution A and solution B.
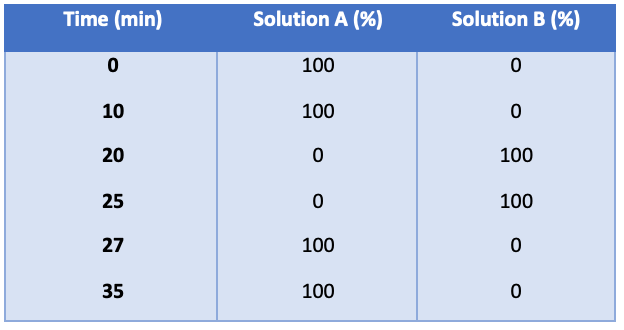
Detector: UV 220 nm
Column: 4.6 mm x 25 cm; 5-mm packing L7 (Octyl Silane (C8) chemically bonded to porous silica)
Column Temperature: 35°
Flow rate: 1.5 mL/min
Injection volume: 20 mL
System Suitability Requirements
Resolution: NLT 3.0 between olanzapine related compound A and olanzapine
Tailing factor: NMT 1.5 for the olanzapine peak
Relative standard deviation (RSD): NMT 2.0% from four replicate injections for the olanzapine peak
Typically, the first decision to make is the new column dimensions that one might like to use and to investigate if the target column dimensions are available with the stationary phase you are using.
The L/dp ratio of the monograph method column is 50,000. So we need column length to particle size ratio columns which lie in the range 37,500 to 75,000.
Some widely available column dimensions which fall within the allowable range include:
- 150 mm x 2.1 mm x 2.7 mm (55,555)
- 100 mm x 2.1 mm x 1.9 mm (52,631)
- 75 mm x 2.1 mm x 1.9 mm (39,473)
- 150 mm x 3.0 mm x 3.5 mm (42,857)
Let’s assume we wish to significantly speed up this method and we select column c) 75 mm x 2.1 mm x 1.9 mm (also assuming that we are using an HPLC system which can handle higher back pressures and is optimized for low dwell volume).
We can then adjust the flow rate to give an equivalent linear velocity for the new column dimensions:

Each segment of the gradient timetable is then adjusted as such:

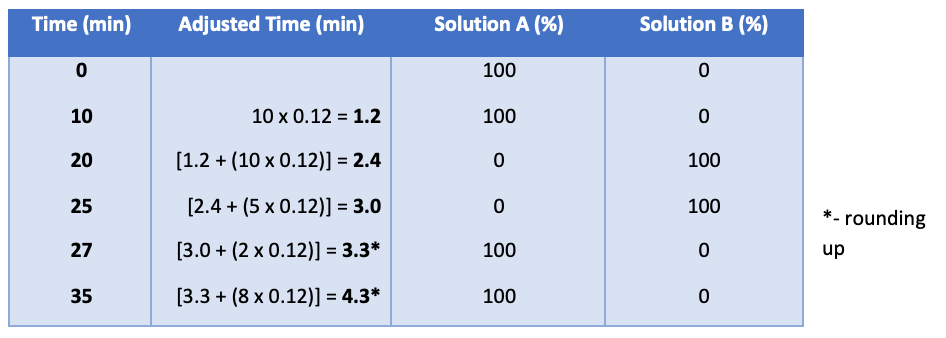
Given the reduction in column volume, it is unsurprising that the gradient times are very much reduced compared to the original monograph method. However, as I mentioned above, one needs to ensure that the system on which the adjusted method is run can repeatably achieve a 100% change in eluent composition in 1.2 min (that is, only very low dead volume, dwell volume systems will be capable of this).
The reduction in column volume will also necessitate a reduction in injection volume, which is calculated as follows:
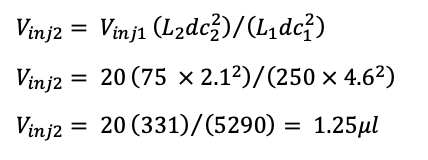
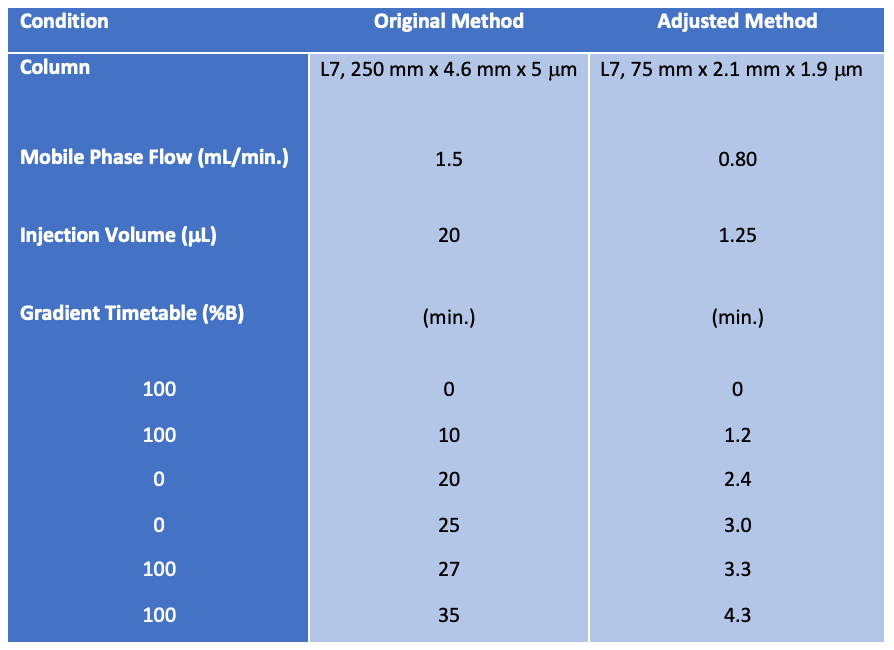
A side-by-side comparison of the adjusted parameters is shown below in Table III.
Table III: A side-by-side comparison of the adjusted parameters.
In practice, the new conditions would be used for the separation and a view taken on how feasible the method is, typically by assessing several repeat injections against the system suitability criteria. If the criteria can be satisfied, and especially where the method is changed to the extent we suggest above, method verification should be performed on the critical criteria of the method. Typically, this might include specificity (especially in the case of adjustments to gradient HPLC methods), selectivity, precision, intermediate precision, and sensitivity (limit of detection), but these are merely suggestions, and one would need to identify the critical criteria for the method under adjustment and the conditions that are altered. Specifically for gradient elution HPLC methods, General Chapter <621> also requires:
- That system suitability criteria are met
- Where the column dimensions are unchanged that the principle peaks elute within ±15% of their respective retention time in the original method
- Any changes to the composition of the mobile phase and/or gradient timetable ensure that the first peaks are sufficiently retained, and the last peaks are eluted
My last point is around the allowable changes to the stationary phase used for the adjusted method in which changes are allowed, provided that the new phase is within the same USP classification (L1, L7, etc.). Most readers will fully appreciate that the selectivity of various phases within these classifications can vary enormously. At the last count, I noted over 200 phases, which purported to fall within the L1 classification! To maximize chances of success, one would be advised to stick to the same manufacturers stationary phase, as undoubtedly the transfer between the different column dimensions and/or particle size and morphology is more likely to be scalable; however, that being said, bonding methods and surface coverage should be checked to ensure that they fall within the allowable changes as outlined within General Chapter <621> (that is, that the surface modification, chromatographic support, and extent of surface modification should be similar). The chapter explicitly states that the change from fully porous to SPPs is allowed, provided that the previous criteria can be met; however, one would need to be mindful regarding the extent and type of surface modification when changing particle morphology as the manufacturer may adjust these parameters between the fully porous and SPPs to optimize column performance.
Of course, if one did wish to change column manufacturer, provided one could satisfy the requirements above, then this is possible. However, to save a great deal of time and heartache, you may wish to refer to one of the several stationary phase characterization tools that exist to help justify this change, and to choose columns which are similar in terms of their primary selectivity characteristics.Such tools are described in the literature (3,4). Working with your column vendor can also help greatly when considering such changes.
It should go without saying that the effort required to verify equivalence of the adjusted method should be very carefully considered when changing between column manufacturers.The potential for retention and selectivity differences is higher and therefore the likelihood of a simple scaling of method parameters as described above, less likely to succeed out of the box.
In summary, the new General Chapter <621> does reflect the great work of the pharmacopeias in helping chromatographers to use more modern technology for their regulated methods, speeding up analysis, and reducing waste. The latest revision allows us to adjust column dimensions for gradient separations, which is a significant advance. Although these freedoms are welcomed, one must be very mindful of the requirements or limits of the allowable changes and the verification work that underpins our demonstration of equivalent performance of the adjusted method. Although in theory these changes avoid the necessity for full revalidation of the analytical method, it is often that when making extensive changes to the method, the verification work required will approach that of a full revalidation. The end user must assess the benefits of change versus the work required to prove method performance equivalence.
References
(1) https://www.crawfordscientific.com/chromatography-blog/post/usp-chapter-621-changes
(2) https://www.chromatographyonline.com/view/lcgc-blog-shape-things-come-possible-changes-usp-chapter
(4) https://apps.usp.org/app/USPNF/columns.html
Tony Taylor is the Chief Scientific Officer of Arch Sciences Group and the Technical Director of CHROMacademy. His background is in pharmaceutical R&D and polymer chemistry, but he has spent the past 20 years in training and consulting, working with Crawford Scientific Group clients to ensure they attain the very best analytical science possible. He has trained and consulted with thousands of analytical chemists globally and is passionate about professional development in separation science, developing CHROMacademy to provide high-quality online education to analytical chemists. His current research interests include HPLC column selectivity codification, advanced automated sample preparation, and LC–MS and GC–MS for materials characterization, especially in the field of extractables and leachables analysis.

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.







