Development and Validation of Novel Gas Chromatography (GC) and Thin Layer Chromatography (TLC) Densitometry Methods for the Quantification of Stigmasterol 3-O-β-D-Glucopyranoside (S3G) in Balanites Aegyptiaca Extract: Application to Newly Formulated Balanites Capsules
Balanites aegyptiaca is a commonly used antihyperglycemic in Egyptian folk medicine. The objective of the present study is to develop two chromatographic methods for standardizing the Balanites extract by quantification of its main component, stigmasterol 3-O-β-D-glucopyranoside (S3G). The first method is gas chromatography (GC) where nitrogen was adopted as the carrier gas. The second method was thin layer chromatography (TLC) densitometry where chromatographic separation was established on aluminum plates using chloroform; methanol was used as the mobile phase followed by a densitometric measurement at 254 nm. Validation of the proposed methods was applied per International Council for Harmonization (ICH) guidelines. Both methods were adopted for quantification of S3G in Balanites capsules. The standard calibration curve was established for S3G in the concentration range of 10–130 μg/mL and 0.5–7.5 μg/mL for the GC and TLC methods, respectively. A good linearity with a correlation coefficient of 0.999 was obtained for both methods. Furthermore, the mean percentage recovery was found to be 99.46% for GC and 100.67% for TLC. Good precision was achieved, with relative standard deviation values <1%, and finally, the limits of detection and quantification were 2.51 and 7.59 μg/mL for GC and 0.13 and 0.40 μg/mL for TLC, respectively.
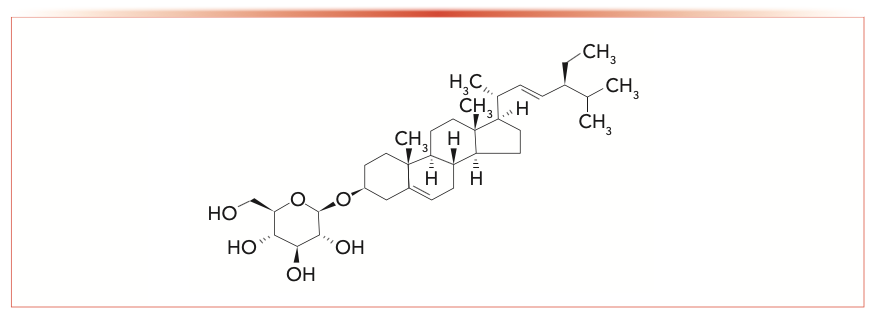
Balanites aegyptiaca, known as the desert date, is a spiny evergreen tree, classified as a member of the Zygophyllaceae family (1). It has been cultivated in Egypt for more than 4000 years, and produces a yellow, single-seeded fruit that is edible but bitter. These fruits are commonly used to treat diabetes in Egyptian folk medicine, and herbalists in the Egyptian market sell the fruits as an antidiabetic drug (2–8). The saponin-rich fraction of Balanites and its content of furostanol saponin derivatives have shown significant in vitro aldose reductase inhibitory activities (9). Several saponins were isolated from the mesocarp of the Balanites fruit (10). Therefore, stigmasterol 3-O-β-D-glucopyranoside (S3G) as a steroid saponin isolated from Symplocos lancifolia is used to standardize the Balanites extract in the present study (Figure 1).
FIGURE 1: Chemical structure of stigmasterol 3-O-β-D-glucopyranoside (stigmast-5, 22-dien-3-O-β-D-glucopyranoside).
The objective of the present study is to develop simple, reliable, and validated analytical procedures for the quantification of S3G by applying gas chromatography (GC) and thin-layer chromatography (TLC). These methods could be used efficiently for routine quality control and for the analysis of Balanites extract both in pure form and in a pharmaceutical formulations. Both GC and TLC densitometric methods were adopted for the first time for quantification of S3G. The GC method was found to be rapid, with minimum sample preparation without the need of any derivatization steps. The TLC densitometry method has the advantage of being relatively inexpensive compared to the GC method used in this study. The literature survey reveals that only spectroscopic analyses, including two-dimensional nuclear magnetic resonance (2D-NMR) techniques and infrared (IR), were previously reported for characterizing the mentioned saponin (10,11).
Experimental
Instrumentation
An Agilent 7890A series GC apparatus (Agilent Technologies) connected with an autosampler was used in this experiment. The output signal was monitored and processed using Chemstation software version B.04.03. An Agilent HP-5 (30 m length × 0.32 mm i.d., 0.25-μm film thickness) column and flame ionization detector (FID) were used. A Camag TLC scanner with winCATS computer software was used in the densitometric method. The TLC plates were pre-coated with silica gel 60 F254 (20 × 20 cm), and a Hamilton 100-μL microsyringe, an ultraviolet (UV) lamp with a short wavelength of 254 nm, and a chromatographic tank (25 cm × 25 cm × 9 cm) were also used.
Materials and Reagents
Pure Samples
Pure S3G was kindly supplied by the Pharmacognosy Department at Cairo University, and the S3G was previously isolated from Balanites aegyptiaca extract with 99.9% purity, assessed by a high performance liquid chromatography (HPLC) method.
Extracts and Market Samples
A Balanites dry extract, in addition to the hard gelatin Balanites capsules (Batch No. Bal0009), were manufactured by Atos for production of medicinal herbs. Each capsule was labeled to contain 400 mg of the Balanites extract (standardized to contain 8.63% of S3G).
Chemicals
For the GC method, chromatographic HPLC grade methanol and acetonitrile (Fischer Scientific) and analytical grade chloroform (Honeywell) were purchased from the local market. An Agilent PTFE 0.45 μm syringe filter was also used.
For the TLC method, analytical grade methanol and ethanol (Adwic), chloroform (Honeywell), sulfuric acid (Sigma Aldrich), and vanillin powder (El-Nasr) were purchased from local market.
A vanillin-sulfuric acid spraying reagent was prepared from 1% vanillin in ethanol (solution I) and 10% sulfuric acid in ethanol (solution II) (12). The plate was sprayed with 10 mL solution I, followed immediately by spraying it with 10 mL of solution II. After heating the plate at 110 °C for 5–10 min under observation, it was evaluated in visible light or daylight.
Standard Solution
Stock standard solution of the drug (0.14 mg/mL) was prepared by dissolving 14.0 mg of S3G in 100 mL 20% methanol in chloroform.
Gas Chromatographic Quantization of S3G
Chromatographic Conditions
The column oven was programmed as follows: The initial column oven temperature was 600 °C and increased to 2800 °C at the rate of 500 °C/min. The injector and detector temperature was kept at 2500 °C and 3000 °C, respectively. Nitrogen was used as carrier gas at a flow rate of 3.35 mL/min and a split ratio at 1:10. The injection volume was 2.0 μL. Methanol was used as the syringe cleaning solvent between injections.
Construction of Calibration Curve
Aliquots of stock solutions of the standard drug (1.0 mg/mL) equivalent to 10.0–130.0 μg/mL of S3G were introduced into a series of 10-mL volumetric flasks. The volume was adjusted using 20% methanol in chloroform, and 2 μL were injected in triplicate for each concentration then chromatographed under the specified chromatographic conditions described previously. The calibration graph of S3G was obtained by plotting the peak area of each concentration against the corresponding concentration.
Application to the Pharmaceutical Dosage Form
The contents of 10 Balanites capsules were mixed well. Then, an aliquot of 593 mg was accurately weighed, dissolved in 100 m of methanol-chloroform mixture in a ratio of (20:80 by volume), and sonicated for 30 min, after which 1 mL from the stock solution was transferred into a 10 mL volumetric flask then the volume was adjusted with the same solvent. The obtained solution was filtered via the syringe filter, and then injected following the specified chromatographic conditions previously described. Three replicate determinations were then made; the corresponding drug concentrations were calculated from the appropriate regression parameters.
The recovery of the procedure was assessed by applying the standard addition technique and the mean percentage recovery (±%RSD) was calculated.
Densitometry Quantization of S3G
Chromatographic Conditions
Analysis was performed on precoated 20 × 20 cm TLC aluminum silica gel 60 F254 plates. Samples were applied to the plates using a Hamilton micro syringe (100 μL). The plates were placed 2 cm apart from each other and 2 cm apart from the bottom edge. The chromatographic tank was presaturated with the mobile phase for 15 min before developing by ascending chromatography using chloroform with methanol (50:50, by volume) as a mobile phase. The plates were then air-dried and sprayed with the vanillin-sulfuric spraying reagent before being heated in the oven at 1100 °C for 5 min. Finally, once detected at 565 nm, all plates were scanned under the following conditions:
- Silt dimensions: 6.0 × 0.3 μm
- Wavelength: 565 nm after spraying
- Scanning speed: 60 mm/s
- Data resolution: 100 nm/step
- Measurement mode: absorption
- Result output: chromatogram and integrated peak area.
Construction of Calibration Curve
Aliquots of drug stock solutions (1.0 mg/mL) equivalent to 0.5–7.5 μg of S3G were introduced into a series of 10 mL volumetric flasks and the volume was adjusted using acetonitrile. Then, 20 μL of each solution were applied to the TLC plates following the specified chromatographic conditions described previously.
Calibration graph of S3G was constructed by plotting the peak area against the corresponding drug concentration in μg/mL.
The Application to the Pharmaceutical Dosage Form
The contents of 10 hard gelatin Balanites capsules were mixed well, and an aliquot of 100 mg of the mixed sample was accurately weighed before being dissolved in 100 mL of 20% methanol in chloroform; this stock solution was sonicated for 30 min. Next, 1 mL of the stock solution was transferred into a 25 mL volumetric flask and completed to volume with acetonitrile This solution was filtered via a syringe filter polytetrafluoroethylene (PTFE) 0.45 μm, and the obtained solution was analyzed following the specified chromatographic conditions described previously. Three replicate determinations were made for each sample. The corresponding drug concentrations were calculated from the appropriate regression model parameters.
The recovery of the procedure was assessed by applying the standard addition technique and the mean percentage recovery (±%RSD) of the added solution was calculated.
Results and Discussion
Method Development and Optimization
In the present study, a simple, rapid, and accurate GC method in addition to a sensitive and validated TLC method were developed for the determination of S3G.
The literature revealed that there is actually no validated GC or densitometric method concerning the estimation of S3G or any other quantification methods. Only a structure elucidation spectroscopic method has been reported.
The described methods in the study could be successfully applied for the analysis of S3G in crude plant sample and future commercial capsules containing Balanites extract.
The proposed methods required minimal sample preparation and an uncomplicated mobile phase.
For the GC method, during the method development, different chromatographic parameters were optimized to obtain an acceptable peak shape and resolution between the peak of solvent and peak of drug with acceptable recoveries to satisfy the GC system suitability.
By trying different flow rates (1–5 mL/min, at constant pressure), it was found that a flow rate less than 3 mL/min causes significant observed peak tailing and that more than 4 mL/min leads to bad resolution between the peaks of the solvent and drug, so a flow rate of 3.35 mL/min was determined to be the best choice.
The split ratio factor was also studied, and it was observed that a high split ratio leads to poor sensitivity, so variable ratios were tried. A 1:10 ratio was selected because it gave good sensitivity and acceptable peak shape.
One of the most important studied factors in this research was the change in column oven heating rate because it was found that low heating rates lead to a peak tailing problem and higher rates (more than 500 °C/min) lead to bad resolution between the peaks of solvent and drug. After studying different heating rates, it was found that 500 °C/min was the best choice.
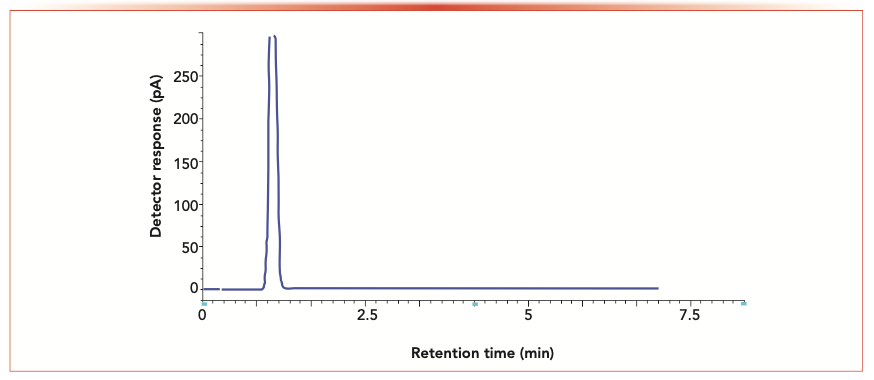
Satisfactory selectivity, sensitivity, resolution, and speed of chromatographic separations with stable baselines were only achieved on a HP-5 column (30 m length × 0.32 mm i.d., 0.25 μm film thickness) using nitrogen as a carrier gas before following the procedure mentioned previously. Applied analysis time was 3 min, as shown in Figures 2 and 3.
FIGURE 2: GC chromatogram of standard S3G.
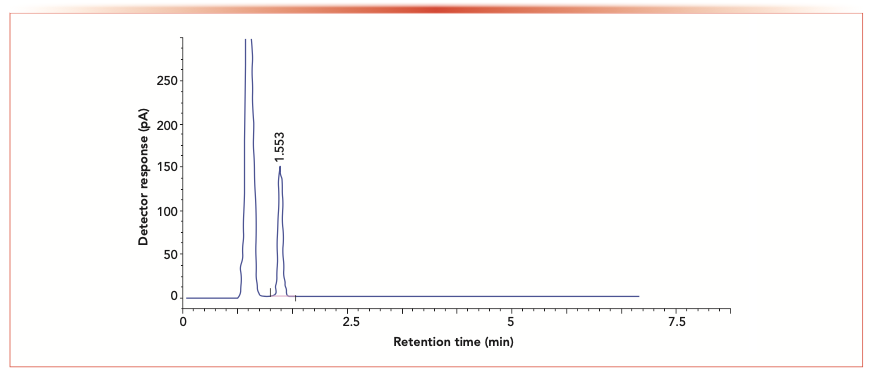
FIGURE 3: GC chromatogram of blank solvent mixture.
For the TLC method, different mobile phases in different ratios were tried, namely hexane:ethyl acetate in a 83:17 ratio, hexane:acetone in a 3:1 ratio (13,14), chloroform:ethanol in a 9.8:0.2 ratio (15), and chloroform:methanol in a 8:0.6 ratio (16).
The best peak shape of S3G was obtained upon using a mobile phase consisting of chloroform:methanol (50:50 by volume).
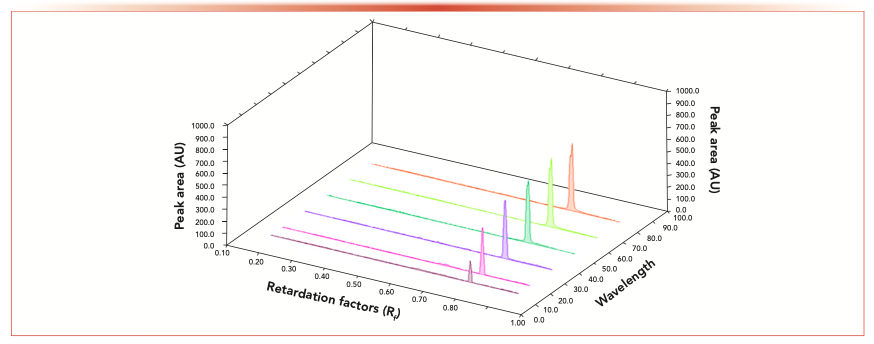
Different scanning wavelengths were tried, including 205, 254, 274, 365, and 565 nm. It was found that the most suitable detection wavelength for S3G was 565 nm after spraying and the spots appeared at Rf 0.78 (Figure 4).
FIGURE 4: TLC densitometry chromatogram of a standard S3G at 565 nm.
Method Validation
The developed method was validated according to the guidelines of the International Council for Harmonization (ICH) for validation of analytical procedures (17).
Linearity and Range
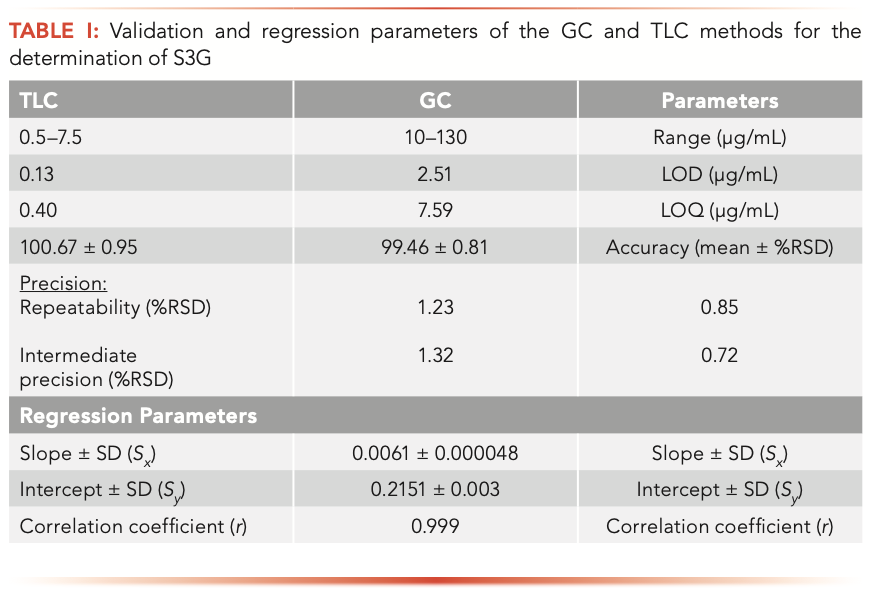
Under the optimized conditions described previously, the analytical curve was obtained by plotting the peak area and the corresponding S3G concentrations over the range of 10–130 μg/mL for GC and 0.5–7.5 μg/mL for TLC. It showed a significant linear relationship and yielded correlation coefficient, r, of 0.999; the regression parameters were computed and presented in Table I.
Limits of Detection (LOD) and Limits of Quantification (LOQ)
The limits of detection (LOD) and limits of quantification (LOQ) were determined based on the standard deviation of the response (σ) and the slope of the calibration curve (S) according to the ICH guidelines, as follows: LOD = 3.3 (σ/S) and LOQ = 10 (σ/S). The LOD and LOQ for GC and TLC were 2.51 and 7.59 μg/mL for the GC method and 0.13 and 0.40 μg/mL for TLC method, respectively, as shown in Table I.
Accuracy and Precision
The accuracy and precision of the procedure was determined using three different concentrations of pure samples of the drug covering the specified range, each in triplicate, within one day for intraday analysis and three days for interday analysis.
To determine the intra- and inter-day precision of the method, S3G was assayed at three different concentrations: (20, 60, and 120 μg/mL) for GC and (1.5, 3.5, and 6.5 μg/mL) for TLC, respectively. The experiment was performed using nine replicates (n = 9) for intra-day and on three separate days in triplicates (n = 9) for inter-day. The %RSD was taken as a measure of precision, and the accuracy of the method was expressed as R%. The results were summarized in Table I.
Repeatability intraday precision of the proposed method for S3G was found to be reliable based on %RSD (<2%).
Intermediate precision (interday precision) was demonstrated on three successive days by two analysts. The %RSD values were less than 2%, confirming that the method is sufficiently precise, as shown in Table I.
Selectivity
The selectivity was assured by studying the possibility of interference of the inactive ingredients in Balanites capsules (placebo) with S3G standard by GC and TLC method. The obtained chromatograms of standard S3G and placebo did not show any peak interference which revealed high selectivity of the proposed method.
Robustness
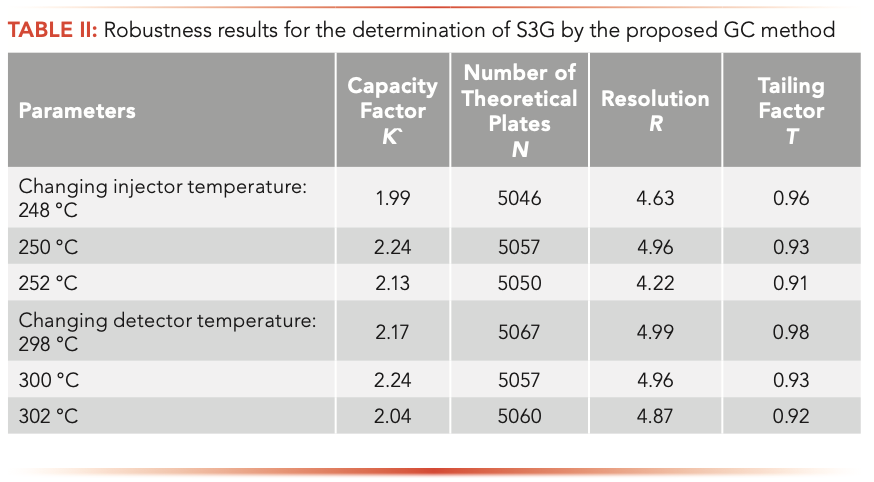
For the GC method, it was evaluated by studying the effect of changing the injector and detector temperature by ±2 °C. It was found that these deliberate variations did not affect the system suitability parameters, confirming robustness of the method and the %RSD found to be <2%, which was also in favor of the developed GC method, as shown in Table II.
For the TLC method, it was evaluated by studying the influence of small variations in the mobile phase ratios (49:51 mL by volume) then observing Rf values upon introduction of these deliberate variations in chloroform and methanol volume.
It was found that these variations cause no significant difference in Rf values. Also, neither tailing of the separated bands or interference were observed, confirming robustness of the method, and the %RSD was found to be 1.41 and 1.53 (<2%) which was also in favor of the developed TLC method.
Stability of Standard Solutions
The stability of the S3G solution in 80% chloroform and 20% methanol was evaluated by analyzing two different solutions: One of them was kept at room temperature, and the other was kept in the refrigerator at 40 °C and compared against the freshly prepared standards. It was found that the drug solution was stable for one week at room temperature and for two weeks in a refrigerator.
Application to Pharmaceutical Dosage Form
The proposed GC and TLC methods were applied for determining S3G in newly formulated Balanites capsules. Three replicate determinations were performed; the obtained results were in good agreement with the label claim, where no interference from excipients and additives was observed, as demonstrated in Tables III and IV.
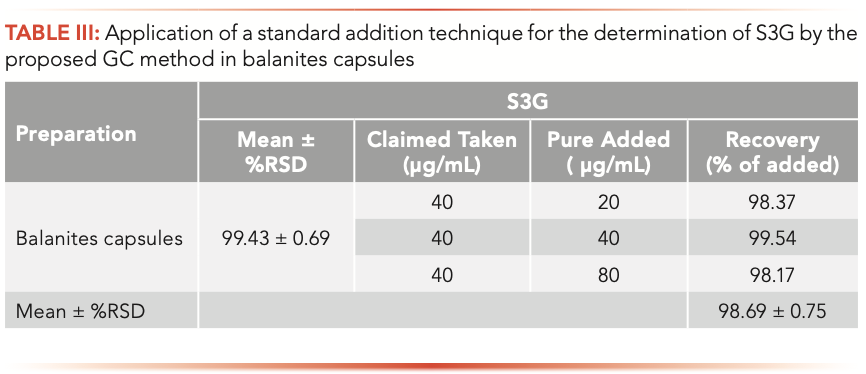
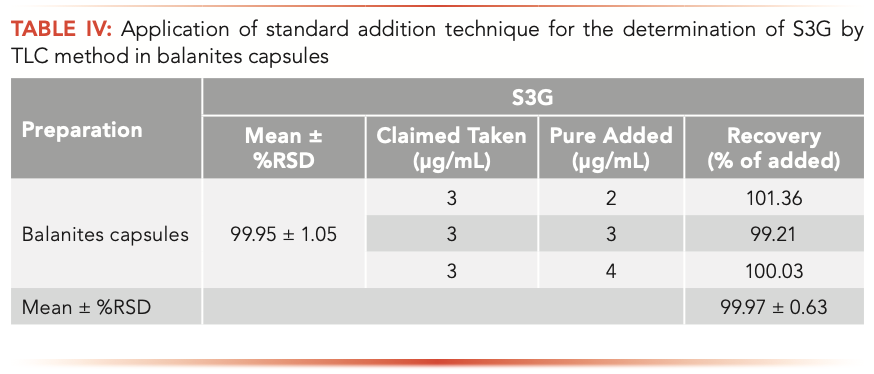
The recovery of the procedure was assessed by applying standard addition technique and the mean percentage recovery ±%RSD of pure added was shown in Tables III and IV.
Conclusion
A novel, simple, rapid and accurate GC method with minimum sample preparation and a wide linearity range, in addition to a newly developed, cost-effective densitometric method, were developed and validated for the estimation of S3G in extracts of Balanites aegyptiaca.
The newly proposed methods are applicable for the determination of S3G in pure form and in pharmaceutical formulations.
References
(1) D.L. Chothani and H.U. Vaghasiya, Pharmacogn Rev. 5, 55–62 (2011).
(2) H. Rashad F.M. Metwally S.M. Ezzat, M.M. Salama, A. Hasheesh, and A.A. Abdel Motaal, Pharm Biol. 55, 1954–1961 (2017).
(3) A.A. Motaal, S. Shaker, and P.S. Haddad, Phcog. J. 4, 20–24 (2012).
(4) M.S. Kamel, K. Ohtani, T. Kurokawa, M.H. Assaf, M.A. El-Shanawany, A.A. Ali., R. Kasai, S. Ishibashi, and O.S. Tanaka, Chem Pharm. Bul. 39, 1229–1233 (1991).
(5) A.E. Baragob, W.H. Almalki, I. Shahid, F.A. Bakhdhar, H.S. Bafhaid, and O.M. Eldeen, Pharmacogn. Res. 6, 1–5 (2014).
(6) N.S. Abou Khalil, A.S. Abou-Elhamd, S.I. Wasfy, I.M. El Mileegy, M.Y. Hamed, and H.M. Ageely, J. Diabetes Res. DOI: 2016:8408326.
(7) N.H. Shafik, R.E. Shafek, H.N. Michael, and E.F. Eskander, J. Chem. Pharm. Res. 8, 128–136 (2016).
(8) A.P. Singh, S. Das, A. Mazumder, M. Kumar, and N. Gautam, J. Pharm. Biol. Sci. 5, 273–277 (2017).
(9) A. Abdel Motaal, H. El-Askary, S. Crockett, O. Kunert, B. Sakr, S. Shaker, A. Grigore, R. Albulescu, and R. Bauer. Phytomedicine 22, 829–836 (2015).
(10) D. Staerk, B.P. Chapagain, T. Lindin, Z. Wiesman, and J.W. Jaroszewski, Magn. Reson. Chem. 44, 923–928 (2006).
(11) Y.C. Teles, O.S. Chaves, M. de Fátima Agra, et al. Rev. Bras. Farmacogn. 25, 363–368 (2015).
(12) H. Wagner and S. Bladt, Plant Drug Analysis: A Thin Layer Chromatography Atlas (Springer Science and Business Media, Berlin, Germany, 2nd Ed., 1996).
(13) B.M. Jaber and S.F. Jasim, Iraqi J. Biotechnology 13, 86–94 (2014).
(14) A. Rishi and S. Sneha, J. Med. Plants Res. 7, 3127–3130 (2013).
(15) K. Kamboj and A.K. Saluja, Int. J. Pharm. Sci. Rev. Res. 19, 60–65 (2013).
(16) A. Kamboj and A.K. Saluja, Arabian J. Chem. 10, 644–650 (2017).
(17) European Medicines Agency, ICH Topic Q2 (R1), Validation of Analytical Procedures: Text and Methodology (Canary Wharf, London, United Kingdom, 2005).
Sara A.A. Elbahrawy is with the Pharmaceutical Research and Development department at Heliopolis University for Sustainable Development in Cairo, Egypt. Amira Abdel Motaal is with the Pharmacognosy Department at Cairo University in Cairo, Egypt, and the Pharmacognosy Department at King Khalid University in Abha, Saudi Arabia. Ola M. Abdallah is with the Analytical Chemistry Department at Al-Azhar University in Cairo, Egypt, and the Pharmaceutical Chemistry Department at Egyptian Russian University (ERU), in Badr City, Egypt. Direct correspondence to: olamody@yahoo.com.

AOAC International Awarded NIST Grant for Developing Drug Testing Standards
October 31st 2024The grant will be part of a new collaborative scientific initiative to address the need for standards that define the desired performance of lateral flow immunoassay test strips to detect illicit drugs in tablets and powders.
Supercritical Fluid Chromatography for Separating Chemotherapy Anti-Nausea Medication
October 30th 2024Scientists from Peking Union Medical College & Chinese Academy of Medical Sciences created a new method for separating palonosetron hydrochloride, which is frequently used to prevent acute or delated nausea and vomiting caused by chemotherapy.
HILIC Peptide Retention Times Predicted Using New Approach
October 29th 2024Manitoba Centre for Proteomics and Systems Biology scientists produced a new means of predicting peptide retention times for hydrophilic interaction liquid chromatography (HILIC) at acidic pH in formic-acid based eluents.