Determination of 25-mono-hydroxy-vitamin D2 and D3 in Human Plasma Using a Symbiosis Pico System: ACN Free On-line SPE-LC–MS–MS
LCGC Asia Pacific
Spark Holland Application Note
Introduction
The method developed runs on a fully automated platform integrating solid-phase extraction (SPE) and LC (Symbiosis Pico) without any manual pretreatment step(s). Automatic pretreatment results in high throughput, low CVs and high precision. Sample preparation has been simplified to just one operator step before the system takes over the automated analysis of the samples.
Instrumentation
A Spark Holland Symbiosis PICO system with a Mistral column oven and an Applied Biosystems/MDS Analytical Technologies API 4000 LC–MS–MS system are used for this application. The API 4000 is equipped with a Heated Nebulizer (APCI) probe.
Symbiosis Pico is Spark Holland's unique (u)HPLC solution with integrated on-line SPE-LC–MS automation. The system offers large flexibility in processing different types of samples selecting one of the three fully automated operational modes LC; XLC (extraction liquid chromatography) and mXLC (multidimensional on-line SPE)
Experimental
Sample preparation consists of diluting a human sample with a special protein disrupting buffer, followed by mixing. Samples are then placed in the Symbiosis autosampler. 100 µL of sample is injected on a Spark HySphere C8EC-SE sorbent SPE cartridge for on-line sample clean-up and concentration.
The mobile phase consists of formic acid, water and methanol with separation on a Merck KGaA Chromolith SpeedROD RP-18e (endcapped), 50 × 4.6 mm HPLC column. An example chromatogram of the separation achieved is shown in Figure 1.
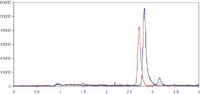
Figure 1: Chromatogram representing 100 ng/mL 25-mono-hydroxy-vitamin D3 (red) and 25-mono-hydroxy-vitamin D2 (Blue) spiked in human plasma.
Results
A calibration curve was created by injecting a full set of calibration standards ranging from 5 to 500 ng/mL spiked human plasma. The calibration curve is calculated with a R2 of 0.999 with a 1/X weighting for both compounds.

Table 1: Accuracy and precision of the QC samples.
Conclusion
This method was evaluated using spiked human sodium heparin plasma samples. The developed on-line SPE-MS–MS method has an absolute recovery of more then 90%, and calibration curve with a linear range from 5–500 ng/mL (R = 0.999 for both compounds). The developed method has an accuracy ranging from 89–117% and precision of 0.1–7.9 %CV. The estimated quantification limits for both analytes are more than sufficient to allow the analytical method to be used for therapeutic drug monitoring. When injecting 100 µL, containing 50 µL of plasma, a detection limit of 5 ng/mL is achieved.
References
1. Spark application note 0053-080-01.
2. Applied Biosystem Vitamin D i-method (User Contributed i-method by Spark).
For more information please send an e-mail to: Applications@sparkholland.com
Spark Holland bv

P. de Keyserstraat 8, PO box 3887800 AJ Emmen, The Netherlands
E-mail: Applications@sparkholland.com
Website: www.sparkholland.com

AOAC International Awarded NIST Grant for Developing Drug Testing Standards
October 31st 2024The grant will be part of a new collaborative scientific initiative to address the need for standards that define the desired performance of lateral flow immunoassay test strips to detect illicit drugs in tablets and powders.
AI and GenAI Applications to Help Optimize Purification and Yield of Antibodies From Plasma
October 31st 2024Deriving antibodies from plasma products involves several steps, typically starting from the collection of plasma and ending with the purification of the desired antibodies. These are: plasma collection; plasma pooling; fractionation; antibody purification; concentration and formulation; quality control; and packaging and storage. This process results in a purified antibody product that can be used for therapeutic purposes, diagnostic tests, or research. Each step is critical to ensure the safety, efficacy, and quality of the final product. Applications of AI/GenAI in many of these steps can significantly help in the optimization of purification and yield of the desired antibodies. Some specific use-cases are: selecting and optimizing plasma units for optimized plasma pooling; GenAI solution for enterprise search on internal knowledge portal; analysing and optimizing production batch profitability, inventory, yields; monitoring production batch key performance indicators for outlier identification; monitoring production equipment to predict maintenance events; and reducing quality control laboratory testing turnaround time.