Not (Only) Reversed-Phase LC–MS: Alternative LC–MS Approaches
In many applications, LC–MS approaches using HILIC, SFC, SEC, IEC, and HIC offer advantages over conventional reversed-phase LC–MS. The examples presented here should inspire you to consider these options for your analyses.
Electrospray ionization (ESI) and other ambient ionization techniques have allowed a successful interface between liquid chromatography (LC) and mass spectrometry (MS). The coupling of these two high-resolution techniques has fostered the use of analytical science in various fields, including, but not limited to, the clinical, pharmaceutical, and forensic fields, enabling the analysis, identification, and characterization of thousands of molecular components in a large diversity of complex mixtures. For many years, reversed-phase LC has remained the most commonly adopted chromatographic mode, due to its rather straightforward applicability to the analysis of a wide variety of compounds (from small to large molecules), as well as its direct compatibility with ESI-MS. However, reversed-phase LC–MS has shown relevant limitations in a number of analytical applications. This encouraged the development of alternative MS-compatible chromatographic techniques, including hydrophilic interaction chromatography (HILIC), supercritical fluid chromatography (SFC), size-exclusion chromatography (SEC), ion-exchange chromatography (IEC), and hydrophobic interaction chromatography (HIC), which provide analyte separation in the liquid phase based on different retention mechanisms compared with reversed-phase LC. Here, we present these alternative chromatographic approaches, highlighting the recent relevant applications in various fields, and discussing their potential in future of analytical science investigations.
Mass spectrometry (MS) enables the structural characterization of molecular components of samples, allowing for the determination of their mass and elemental formula. Both intact mass and fragmentation pattern (such as using tandem MS/MS experiments, for example) lead to unique molecular information, which is crucial for compound identification in a wide variety of targeted and untargeted applications, from forensic research to synthetic polymer characterization.
Direct MS experiments (such as flow injection MS and ambient ionization techniques such as paper spray MS) are high-throughput approaches where the analytes are directly ionized and detected without prior chromatographic steps. Yet, direct MS is typically not suited for the analysis of complex samples due to possible matrix effects and relatively narrow dynamic range, which can significantly decrease the amount of information that is gathered per sample.
The marriage between liquid chromatography (LC) and MS has been made possible by the introduction of atmospheric ionization interfaces, notably electrospray ionization (ESI) (1,2). With LC–MS, the matrix effects can be reduced, the dynamic range significantly extended, and the sample components concentrated (3,4). Remarkable results in terms of sensitivity have been reported. For instance, identification of sub-zeptomolar (zM) peptides using low-flow separations (pL/min) allowed for the determination of the protein content in a small number of cells (5–8).
The research publication output of LC–MS and LC–MS-based studies between 1970 and 2019 is illustrated in Figure 1a. The steady growth of both LC and MS is accompanied by a steep increase in the percentage of publications using LC–MS (plotted as a function of LC publications). This trend is especially visible starting from the late 1990s, when LC–MS platforms became commercially available and, with time, developed to more and more robust instruments.
Figure 1: (a) Number of publications per year in LC (dark blue), MS (red) and %LC–MS (black) literature between 1970 and 2019. Results have been extracted from Scopus using the following search: “liquid chromatography”, “mass spectrometry” and “LC–MS” as keywords. The result on the right y-axis “%LC–MS (vs. LC publications)” are obtained by calculating the percentage of LC–MS publications with respect to the LC publications in the same year. (b) Overview of the chromatographic modes used in LC–MS analysis. Results for reversed-phase LC–MS have been retrieved from Scopus using the following search: RPLC–MS (TITLE-ABS-KEY (liquid AND chromatography AND mass AND spectrometry) AND TITLE-ABS-KEY (reversed-phase AND chromatography) AND (LIMIT-TO [DOCTYPE, “ar”]) AND (LIMIT-TO (SUBJAREA, “CHEM”). Similarly, the same filters have been applied for the other chromatographic modes with the exception of the descriptor “TITLE-ABS-KEY” which has been replaced by “hydrophilic-interaction” for HILIC–MS, ”ion-exchange” for IEC–MS, “size-exclusion” for SEC–MS, “normal-phase” for NPLC–MS, “supercritical-fluid” for SFC–MS, and “hydrophobic-interaction” for HIC–MS.
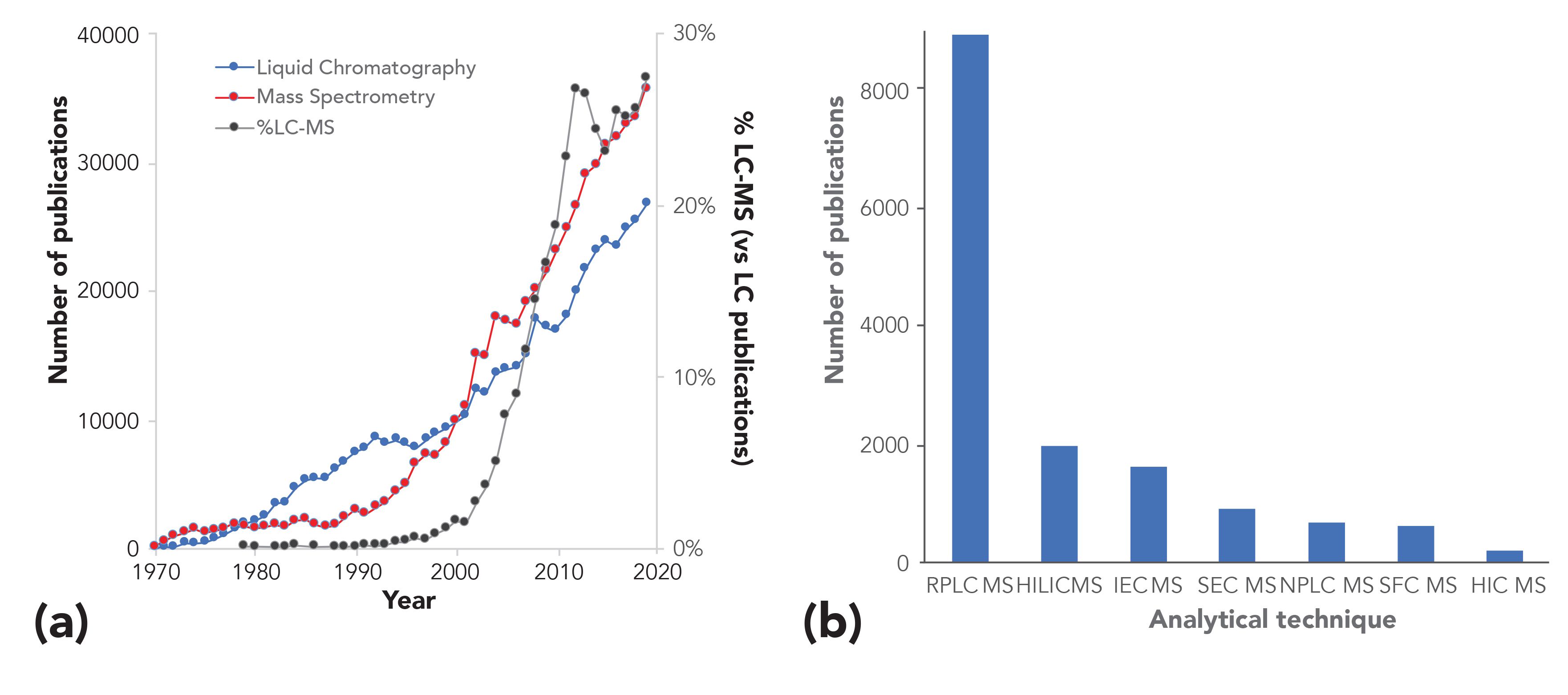
Although the results shown in Figure 1a are mostly reflecting academic research trends, a similar development has also been observed in industries and clinical laboratories, where an increasing number of LC–MS methods have been introduced in R&D departments, for end-product characterization as well as (although less frequently) for quality control purposes (9,10).
Since the introduction of the first commercial LC–MS instruments in the 1990s, numerous technological developments have been carried out in this field, further expanding the separation resolution. The advent of ultrahigh-pressure liquid chromatography (UHPLC) and high-resolution mass spectrometry (HRMS) has unquestionably revolutionized the field of LC–MS. Besides the commercialization of columns equipped with sub-2 µm particles, the development of columns equipped with core-shell particles and, more recently, micropillar array columns have brought new exciting perspectives in terms of separation efficiency, significantly decreasing peak widths and increasing the peak capacity of LC–MS separations.
Many of the developments carried out in LC–MS have focused on reversed-phase LC–MS. Indeed, reversed-phase LC very often remains the starting method for most of the investigations targeting compounds with good solubility in water-organic mixtures. The reason for this choice is explained by its robustness, high separation efficiency, and simplicity in the coupling with ESI and other ambient ionization interfaces. This is reflected in the much higher number of studies using reversed-phase LC–MS published since 1970, compared to all other chromatographic techniques, as shown in Figure 1b.
However, the concept of “one-size-fits-all” does not always apply in analytical science. Indeed, in many cases, reversed-phase LC–MS may not offer the selectivity or resolving power needed for a given analytical investigation. This review showcases a selection of relevant technological developments and applications coming from different areas, such as analysis of small molecules, proteins, and synthetic polymers. In these applications, the coupling of alternative chromatographic techniques—namely, hydrophilic-interaction chromatography (HILIC), supercritical fluid chromatography (SFC), size-exclusion chromatography (SEC), ion-exchange chromatography (IEC), and hydrophobic interaction chromatography (HIC)—to MS has proven to offer advantages over the conventional reversed-phase LC–MS-based approaches. These examples of alternative LC–MS developments and applications have been carefully selected to further inspire analytical scientists, and provide them with additional options to solve a large diversity of analytical questions.
Hydrophilic Interaction Chromatography (HILIC)
HILIC–MS is considered the second most common chromatographic mode used in LC–MS analysis (Figure 1b). The reasons for its widespread diffusion include an orthogonal selectivity to reversed-phase LC, allowing for the retention of polar-ionizable compounds often poorly retained in reversed-phase LC (11), a lower mobile phase viscosity (enabling the use of longer columns leading to higher efficiencies), and a high sensitivity when using ESI-MS due to the use of high organic percentages in the mobile phase (12,13).
A large number of papers in the literature have investigated HILIC retention mechanisms, describing the multimodal nature of this chromatographic mode (14). The retention is driven by the partition of analytes between a polar stationary phase and a relatively hydrophobic mobile phase (for example, an aqueous–organic mixture containing a high proportion of acetonitrile). Under appropriate conditions, namely, a concentration of 5 to 40% water in the eluent, a water-enriched layer is formed at the surface of the stationary phase, allowing for the hydrophilic partitioning. HILIC retention also involves ionic interactions, dipole–dipole interaction, and hydrogen bonding (15,16). The stationary phases can be classified on the basis of their chemistry as neutral, such as diol and amide, and charged stationary phases, such as bare silica, zwitterionic, and amine (17).
The analyte retention strongly depends on the selection of the stationary phase chemistry, as well as the buffer composition used in the mobile phase. Moreover, in order to ensure reproducible experiments, careful attention has to be paid to essential operating parameters, such as the composition of the injection solvent and the use of a reproducible mobile phase composition, as well as adequate equilibration time between runs. We refer to published literature for more insights on method development in HILIC–MS (18,19).
HILIC–MS has significantly matured over the last years, along with the commercialization of columns ensuring an improved batch-to-batch reproducibility and the introduction of stationary phases equipped with sub-2-µm particles. HILIC–MS is currently used in a wide range of fields, including pharmaceutical analysis, metabolomics, lipidomics, and glycomics. Moreover, recent investigations have demonstrated the suitability of HILIC–MS to study large molecules, such as intact proteins and their subunits, making it an attractive orthogonal tool for the analysis of glycosylated biotechnological products.
From Metabolomics to Lipidomics
One of the major challenges in metabolomics is the wide diversity in physico-chemical properties observed between metabolites, showing the need for complementary approaches enabling a larger coverage of the metabolome. The human metabolome encompasses a large number of (highly) polar metabolite classes such as amino acids, small organic acids, nucleosides, nucleotides, and phosphate derivatives, as well as saccharides, which are playing key roles in multiple (patho)physiological processes. Such metabolites show poor retention, and suffer from severe matrix effects using reversed-phase LC, leading to poor quantitative accuracy and low sensitivity. However, they are typically well-retained using HILIC. HILIC–MS also typically leads to a broader metabolome coverage in untargeted metabolomics compared with reversed-phase LC–MS (14,20). For all these reasons, HILIC–MS is now considered by the metabolomics community as essential as reversed-phase LC–MS, being integrated in the state-of-the-art analytical toolbox.
One of the major challenges in HILIC-based metabolomics is to develop methods offering a good compromise between metabolome coverage and acceptable peak shapes for different metabolite classes. The presence of broad peaks results in additional challenges during data pre-processing that may lead to poor quantitative accuracy. Better peak shapes for all compounds can be obtained by using multiple HILIC methods in successive experiments. However, this strategy is often not possible, due to time constraints and sample or resource availability. Most reported applications have therefore been carried out using one single HILIC method; for example, using a zwitterionic or diol column in untargeted metabolomics approaches, sacrificing part of the coverage for a better analytical efficiency.
Peak broadening has been significantly reduced by adding micromolar concentrations of phosphate to the mobile phase buffer (5 μM phosphate, corresponding to an estimation of ca. 40 nmol introduced to the column during each run). In the presence of trace amounts of phosphate, a significantly better peak shape, signal intensity, and improved coverage have been observed for a set of 65 polar compounds, including neurotransmitters, small organic acids, nucleosides, nucleotides, biogenic amines and sugars (Figure 2) (21). Similar improvements have been observed when a comparable amount of phosphate was added to the sample injection solvent. Moreover, in addition to the chromatographic effects, a slight increase of ionization efficiency has been observed in presence of phosphate (5 μM in the mobile phase), that is., 6% for negatively ionizing compounds and 16% for positively ionizing compounds, respectively. The significant improvement in peak shapes led to a more accurate automatic peak detection, representing a key advantage in the data preprocessing pipeline for untargeted applications. Interestingly, no sign of source contamination or instrument failure was observed after a year of experiments.
Figure 2: Improvement in peak shape observed with addition of trace phosphate in the HILIC mobile phase: (a) epinephrine, (b) isocitrate. Upper red trace, without phosphate; lower green trace, with 5 µM phosphate added to the mobile phase. Separation performed using a Sequant ZIC-pHILIC column (50 × 2.1 mm, 5-μm); mobile phase composition: (1) 20 mM ammonium acetate in water:acetonitrile (95:5, v/v) containing 5 µM ammonium phosphate, and (2) 100% acetonitrile. Adapted from reference (21) with permission.
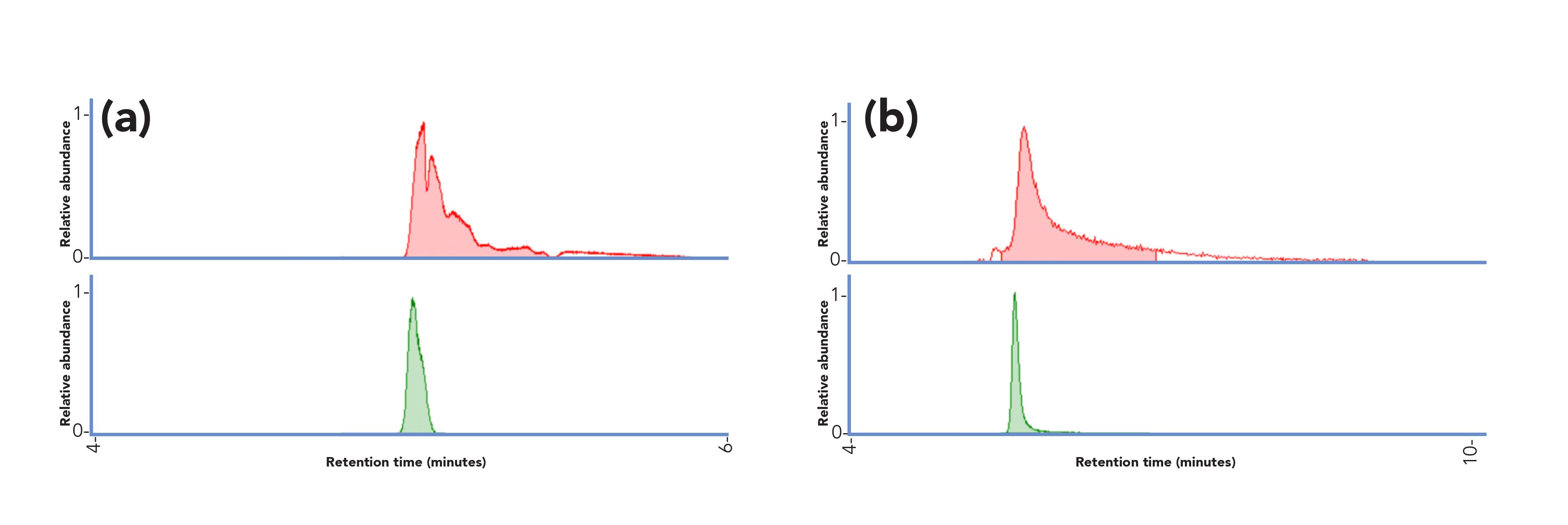
With additional experiments, the authors showed that the presence of trace phosphate improved the chromatographic performance by shielding electrostatic interactions between the analytes and the polymeric-based zwitterionic stationary phase, similar to what is observed when increasing the concentration of salts in the mobile phase. Indeed, a similar behavior in performance enhancement was observed when increasing the concentration of ammonium acetate in the mobile phase (i.e., from 5 to 200 mM) compared with the addition of trace phosphate (21). The analytes being the most positively affected by the presence of phosphate were also the ones requiring a higher concentration of ammonium acetate to achieve optimal peak shape. Moreover, phosphate – which has a high charge density – showed to be highly beneficial to improve the peak shape of compounds whose elution profiles were negatively affected by strong electrostatic interactions.
The exact mechanisms underlying this shielding effect remain to be fully understood, as different effects were observed depending on the column batch used. Indeed, irregularities in column manufacturing – as well as column conditioning – may modify the accessibility of phosphate to the electrostatic sites of the stationary phase. Moreover, phosphate blocks trace metals within the stationary phase matrix, influencing the peak shape. Interestingly, this study not only highlighted the benefits of adding trace phosphate to the mobile phase, but also revealed that electrostatic interactions may be the predominant cause of poor chromatographic performance when using HILIC, notably in metabolomics.
Lipidomics is a subdiscipline of metabolomics focusing on the large-scale study of the structure and functions of lipids. Lipids are involved in a plethora of physiological functions, including energy storage, signaling, and regulation of protein function. More than 60% of all human metabolites have been annotated as lipids (22). Reversed-phase LC–MS is widely used in lipidomics, typically using a C8- or C18-based column combined with a highly organic gradient, allowing for the separation of lipids based on their fatty acyl chain length and unsaturation.
HILIC–MS, on the other hand, offers an orthogonal chromatographic separation where lipids are separated based on the lipid headgroup polarity, however typically leading to the co-elution of all lipids of a specific lipid class (23). The latter is considered advantageous in quantitative analysis, as the target lipids of different classes are co-eluting with their labeled internal standard (ISTD), chosen for each class. In reversed-phase LC–MS, ISTDs are usually eluted at a different retention time, possibly showing different matrix effects than the target lipids. Lange and associates evaluated the quantitative performance of HILIC–MS and reversed-phase LC–MS for the analysis of five lipid classes in human plasma (phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, lysophosphatidylcholine, and lysophosphatidylethanolamine) using the so-called “one ISTD-per-lipid class” approach (23). They demonstrated that, despite the obvious difference in matrix effects, both workflows can be equally used for quantitative analysis, as similar concentrations were measured for most of the lipid classes assessed, consistent with reported NIST consensus values (except for highly unsaturated phosphatidylcholines).
Analysis of Intact or Subunits of (Glyco)Proteins
HILIC–MS has recently emerged as an attractive analytical tool for proteins analysis, in particular for the characterization of glycoproteins. Glycosylation is a common post translational modification (PTM), where oligosaccharides (referred to as glycans) are covalently bonded to an amino acid residue of the protein. Glycoproteins have an heterogeneous glycan composition; differences in their distribution may have a major impact on the biological functions of the protein and its stability, solubility, antigenicity, folding, and half-life (24,25). The glycosylation pattern is therefore considered a Critical Quality Attribute of recombinant pharmaceutical proteins, and should be carefully monitored in drug development pipelines to ensure quality, safety, and efficacy of the biopharmaceutical product.
Similar to what is observed for small molecules, HILIC allows for the discrimination between protein sample components based on a mechanism orthogonal to reversed-phase LC (26). In particular, optimized HILIC–MS methods have led to the separation of glycoforms of glycoproteins, such as monoclonal antibodies (27,28) and their fragment crystallizable portion (29), biopharmaceuticals (30), and neo-glycoproteins (31).
In most cases, protein samples are solubilized in water–acetonitrile mixtures, typically high in water content and containing an acidic ion-pair (such as trifluoroacetic acid). Small volumes (<10% of column volume) of this solution are injected and analyzed using acetonitrile–water gradients on amide stationary phases. The mobile phase conditions allow for ion-pair formation, with the basic protein residues at low pH reducing ion-exchange interactions with the stationary phase. As a result, the (neutral) sugars of protein glycans substantially contribute to the retention, resulting in glycoform resolution based on the overall glycan size and composition.
Figure 3 shows an example of the resolving power that can be obtained with HILIC–MS for the characterization of a lipase enzyme used in the food industry (results obtained in our laboratory) (32). Whereas the reversed-phase LC–MS analysis resulted in a single peak, the HILIC–MS method revealed the different glycoforms of the glycoprotein, resolved into distinct peaks according to the number of glycose units and the glycosylation site occupied (Figure 3). The HILIC–MS method resulted in the detection of over 100 glyco-proteoforms, allowing for the detection of glycoforms with two and three glycosylation sites occupied that were not observed with reversed-phase LC–MS. Interestingly, the total ion current profile obtained was similar to the SDS-PAGE densitogram. Off-line fractionation of the HILIC-UV separation confirmed this observation.
Figure 3: HILIC–MS analysis of a lipase enzyme. (a) Total ion current (TIC) with annotated glycoform elution windows (xN-yM), where (x) indicates the number of N-glycosylation sites occupied (0–3) and (y) the number of mannose units present next to a single N-acetylglucosamine present in each glycan. (b) Deconvoluted mass spectra obtained for the corresponding elution windows (7.5–7.7 min, 8.7–8.8 min, 8.8–9.0 min and 9.0–9.3 min), showing signals for the mature form of the protein (intact sequence, Ma), a mature form without a C-terminal tryptophan (Ma-W) and a mature form without a C-terminal serine and tryptophan (Ma-SW). (c) SDS-PAGE of the lipase showing the derived densitogram (top trace) aligned with the TIC trace obtained with HILIC-MS. Reproduced from reference (32) with permission.
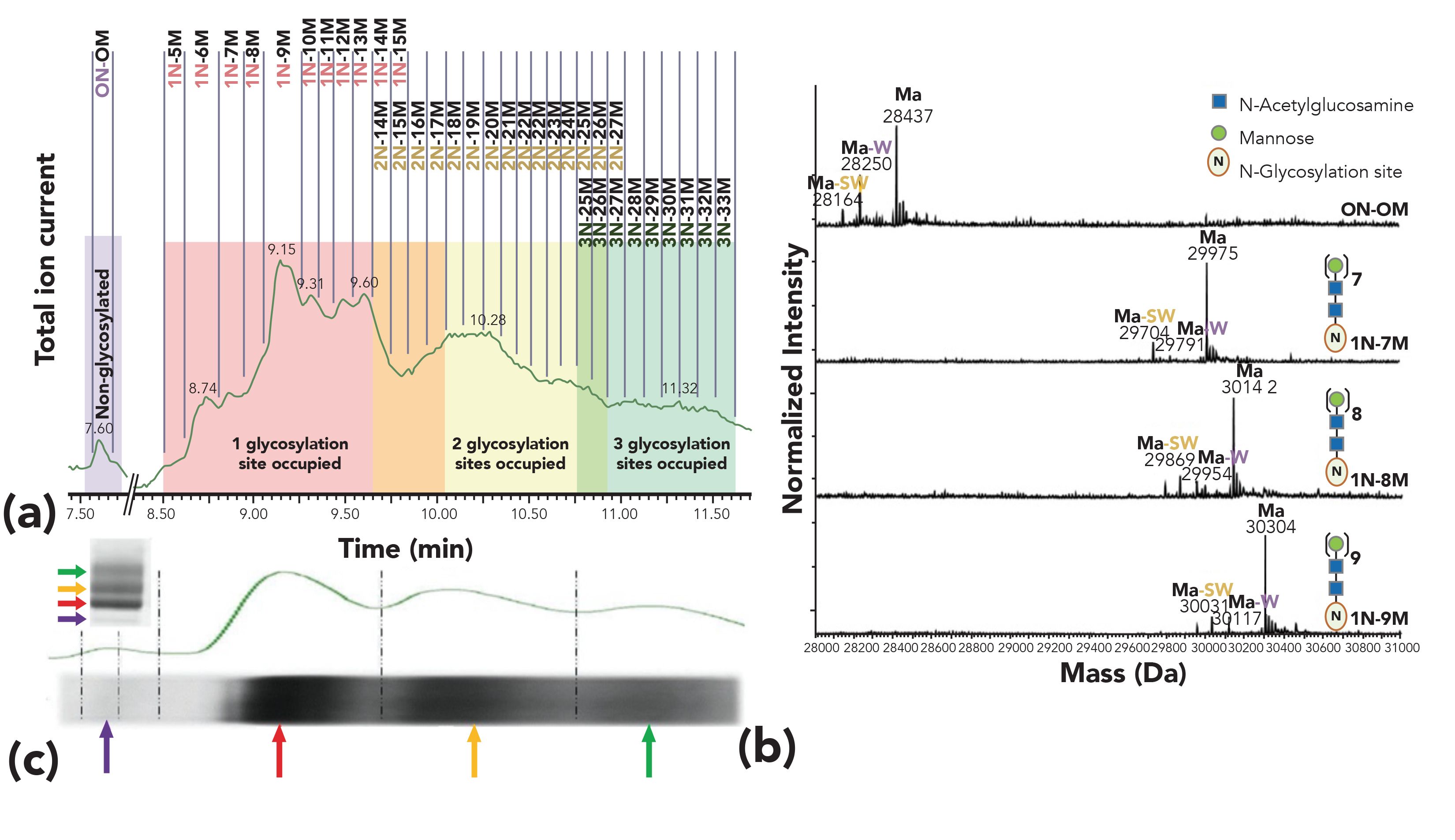
An important aspect in HILIC–MS being currently actively investigated is the replacement of ion-pair additives, such as trifluoroacetic acid, with more MS-friendly acidic ion-pair, such as formic acid, to increase the sensitivity of the method while maintaining its selectivity. In this context, an interesting development has been presented by the group of Wirth and co-workers, who used stationary phases with a special polymer coating to reduce the ionic interactions between the stationary phase and the proteins, preserving the same HILIC selectivity while significantly reducing the concentration of trifluoroacetic acid needed (33–35).
Supercritical Fluid Chromatography (SFC)
First introduced in the last century with minor success, supercritical fluid chromatography (SFC) has regained a substantial popularity in recent years due to the remarkable technology advances carried out in modern SFC instrumentation, which have greatly improved its reliability, reproducibility, and robustness (36). SFC is based on the use of supercritical fluids as mobile phase, typically carbon dioxide (CO2), which is considered the solvent of choice, due to its low critical temperature (31 °C) and critical pressure (73.8 bar). Due to the low polarity of CO2, a small proportion of a miscible organic modifier, usually methanol, is added to the mobile phase, extending the application of SFC to the analysis of more polar compounds. Compared with HPLC, the high proportion of supercritical fluids in the mobile phase offers a faster mass transfer thanks to an enhanced diffusivity. Moreover, the low viscosity of the SFC mobile phase leads to a lower pressure drop, which allows for the use of significantly higher flow rates compared with HPLC. Besides the organic modifiers, acids, bases, or salts at low concentrations, as well as water, can be added to the mobile to increase selectivity and separation efficiency, improve peak shape, and enable the elution of polar compounds (37). However, the presence of a modifier and additives in the mobile phase influences the critical parameters of the fluid, which is then closer to the so-called subcritical conditions (38).
Most of the reversed-phase, normal-phase, and HILIC stationary phase chemistries can be used in SFC, overall offering a wide selectivity range (39). Moreover, multiple SFC-specific stationary phases have been developed and commercialized over the last years. A large number of the commercially available columns are also available with fully porous sub-2-µm and superficially porous sub-3-µm particles, enabling ultrahigh-performance SFC (UHPSFC) analysis which leads to excellent kinetic performance with a low pressure drop compared with UHPLC (40,41).
Hyphenating SFC with MS remains more challenging than the rather straightforward LC–MS configuration, due to the depressurization of the mobile phase which may lead to compound precipitation and cooling effects (36). Many approaches have been recently proposed to interface SFC with MS, with two designs currently available on commercial instruments, namely, the pre-backpressure regulator (pre-BPR) splitter with sheath pump, and the backpressure regulator (BPR) and sheath pump with no splitter interfaces, both illustrated in Figure 4a (42). In the pre-BPR splitter with sheath pump interface, the effluent is mixed with an added make-up solvent flow, and split into two parts, with the smaller proportion directed to the MS, and the remaining larger proportion directed to BPR to maintain the adequate pressure in the system. In the BPR and sheath pump with no splitter interface, the effluent is mixed with the make-up solvent flow, and directed to the MS inlet placed after the BPR. Adding a make-up flow to the SFC–MS interface has two purposes: enhancing the ionization efficiency, and avoiding a possible precipitation along the tubing. With such interfaces, the conventional ESI and atmospheric-pressure chemical ionization (APCI) sources can be used. Compared to LC–MS, SFC–MS typically shows higher sensitivities, due to an improved evaporation process, as a rapid evaporation of CO2 occurs at the MS inlet, which leaves the analytes in a small amount of easily-evaporated organic modifier (42). Finally, most of the common mass analyzers used in LC–MS, such as single quadrupole, triple quadrupole, and time-of-flight, as well as hybrid instruments are also suited for SFC–MS analysis (41,43,44).
Figure 4: Hyphenation of SFC with MS. (a) The two commercially available interfaces used to hyphenate SFC with MS (pre-BPR splitter with sheath pump interface and BPR and sheath pump with no splitter interface). (b) UHPSFC–MS interface design developed to improve the spray stability. Note: A is commercially available interface and B is the modified prototype interface. Adapted from references (42,45) with permission.
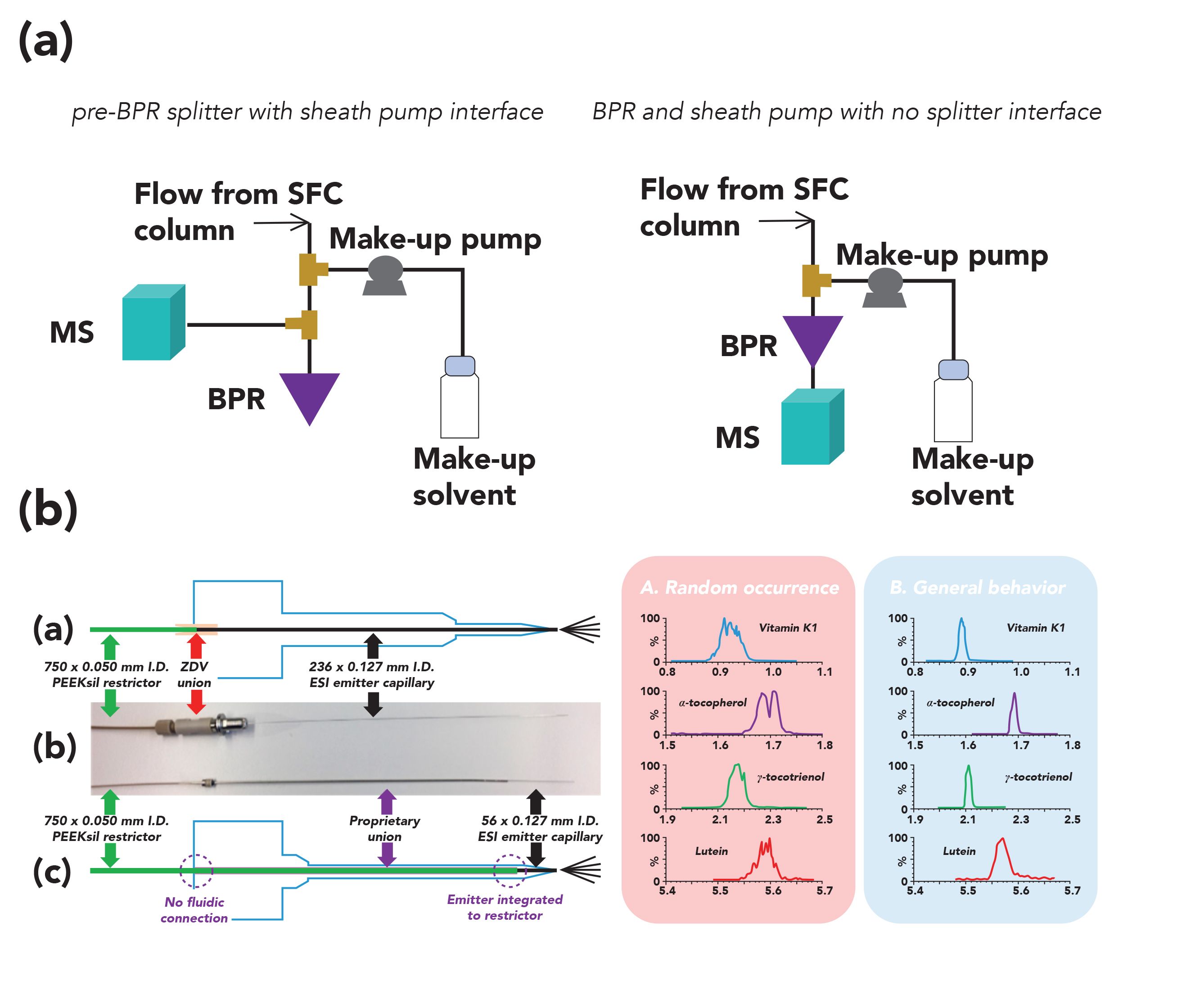
Due to the nonpolar nature of CO2, SFC–MS has been mostly used for the analysis of relatively non-polar lipids, steroids, tocopherols, and vitamins. As an example, a validated workflow for the quantitative analysis of 14 fat-soluble vitamins and carotenoids in human plasma was developed using UHPSFC–MS (45). Using a C18 stationary phase and adding a small proportion of water to the mobile phase, multiple isomers and tocopherols were successfully separated in a single 8-min run. The commercial SFC–MS interface hardware was improved to minimize the post-decompression volume and allow for a better control of the chromatographic effluent density before the ESI process (Figure 4b). Combined with specific make-up solvent conditions, this new prototype interface led to a more stable spray, reducing the occurrences of spiky peaks, and resulting in an improved repeatability and sensitivity.
SFC–MS is not only well-suited for the analysis of hydrophobic compounds, but also fully applicable to the analysis of (highly) hydrophilic compounds using optimal experimental conditions. Moreover, with one of the major advantages of SFC–MS being its great versatility, the simultaneous analysis of both hydrophobic and hydrophilic compounds has become achievable, as demonstrated in recent studies (46–50). As an example, a comprehensive study has demonstrated the applicability of UHPSFC–MS in metabolomics for the simultaneous analysis of both nonpolar and highly polar metabolites within one single run, which remains very challenging using UHPLC-based methods (47). The complete separation of the compounds set composed of 57 metabolites of different polarities (-6 < logP <11) was achieved in a single UHPSFC–MS run, using optimized conditions (a mobile phase gradient ranging from 2% to 100% of modifier, a column packed with sub-3 μm superficially porous particles and a mix of water/acetonitrile [50:50, v/v] as sample diluent) (47). Figure 5a shows the separation of two compounds with a large polarity difference (tricosanoic acid [logP = 9.3] and raffinose [logP = -6.3]), thus demonstrating the suitability of UHPSFC–MS for metabolomics.
Figure 5: SFC–MS for the simultaneous analysis of hydrophobic and hydrophilic metabolites. (a) SFC–MS chromatogram showing the simultaneous injection of tricosanoic acid and raffinose (see reference [47] for experimental conditions); (b) Scatter plot showing the molecular weights of the detected metabolites as a function of their retention times. Blue dots, metabolites detected from the library; red dots, metabolites from the selected experimental set of metabolites. Adapted from reference (47,48) with permission.
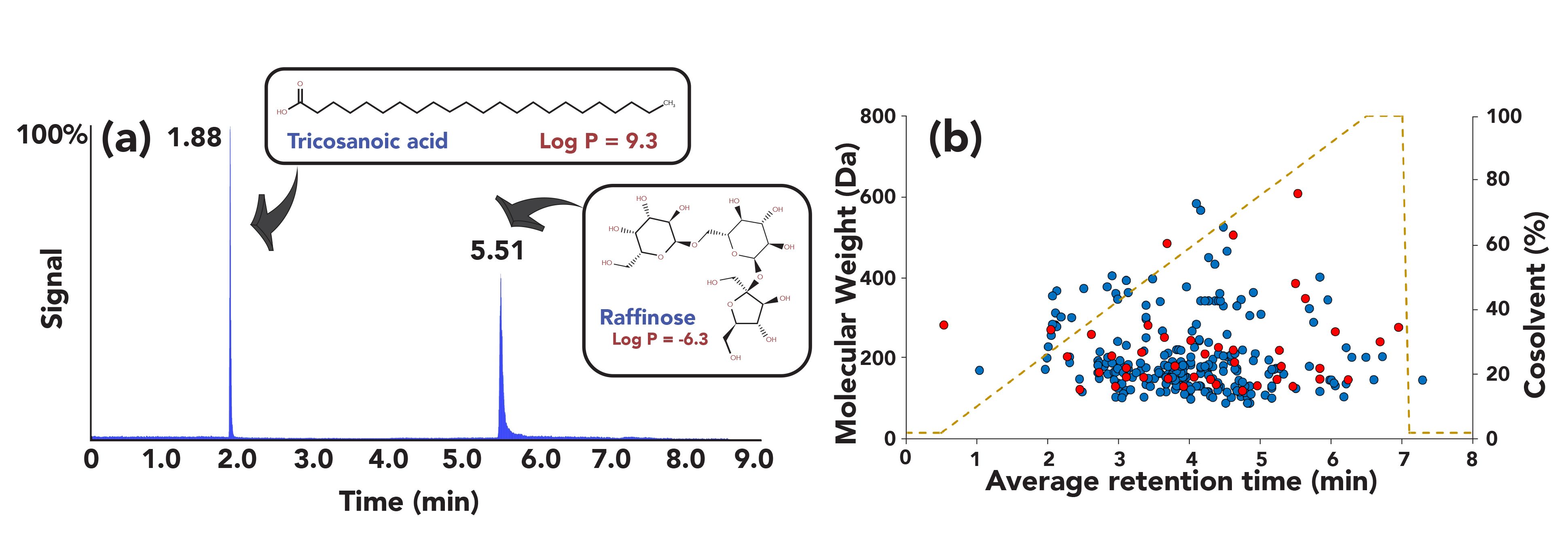
Besides metabolome coverage, another important aspect in metabolomics is the presence of matrix effects, which may significantly affect the quality of the quantitative data and represent a crucial parameter during method optimization. In their study, Guillarme and coworkers also investigated the importance of matrix effects in plasma and urine for a set of representative metabolites (48). Overall, limited matrix effects were observed for the analysis of both biological fluids, with 30% of the metabolites suffering from matrix effects in plasma and 25% in urine, respectively. Moreover, the repeatability of the UHPSFC–MS coupling was remarkable, with average relative standard deviation (RSD) values for retention time repeatability between 0.3 and 0.5% over a period of three weeks. The authors also evaluated the performance of UHPSFC–MS in metabolomics using a commercial library containing 597 metabolites. With UHPSFC–MS, 66% of the compounds present in the library were detected, notably highly polar compounds such as amino acids, nucleosides, and carbohydrates, as well as hydrophobic analytes such as steroids and lipids (Figure 5b). Phosphorylated metabolites, however, led to poor performance.
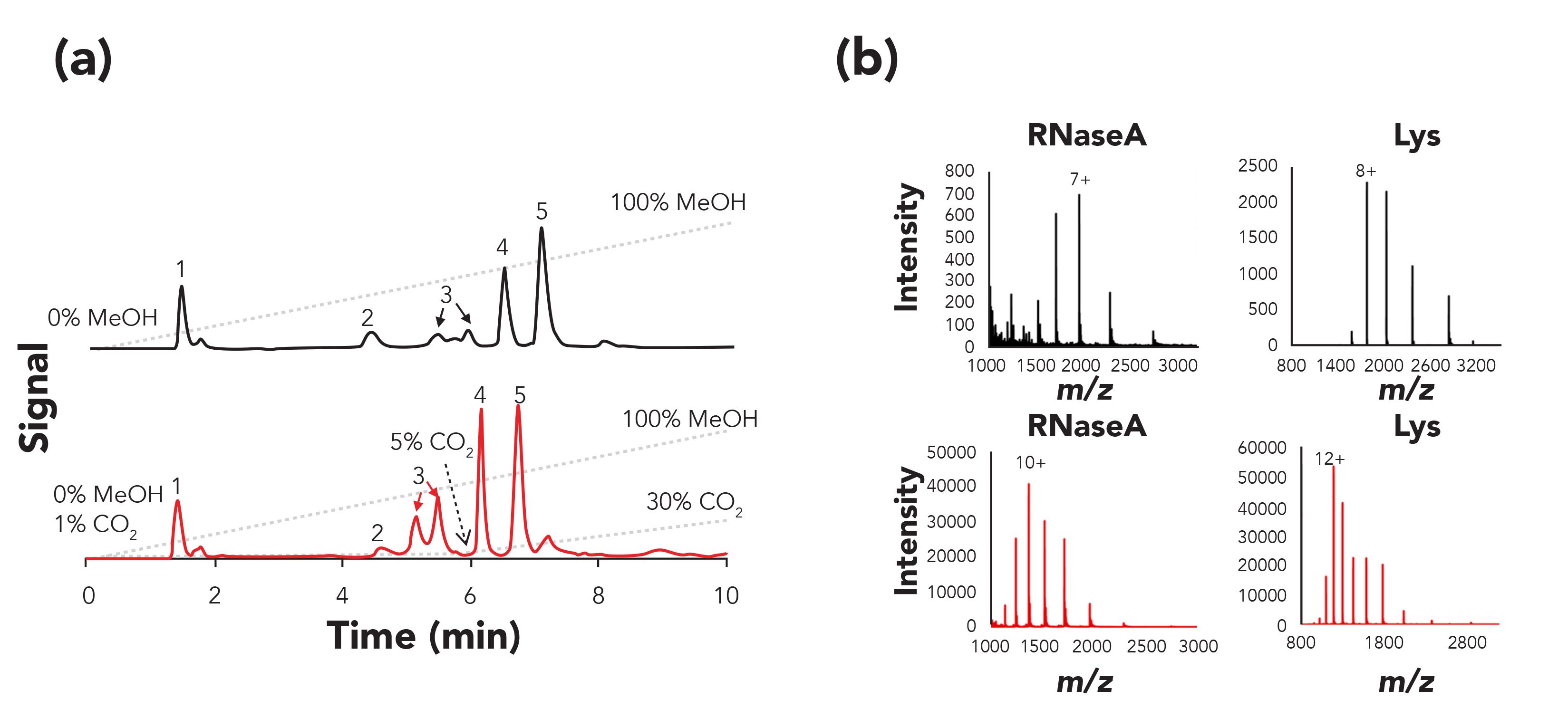
SFC–MS does not only show interesting advantages for the analysis of low-molecular weight compounds, but has also demonstrated a great potential for the analysis of proteins. As an example, SFC (here referred to as enhanced fluidity liquid chromatography by the authors) was used to separate intact proteins using hydrophobic interaction chromatography stationary phase combined with a dual gradient elution that consisted of an LC solvent gradient with simultaneous addition of an increasing amount of CO2 (51). Compared to conventional LC analysis, improved efficiency was observed using SFC, due to the faster mass transfer caused by the presence of CO2 (Figure 6a). As the desolvation of the mobile phase in the MS was also enhanced by the addition of CO2, a “supercharging” effect was also observed, resulting in higher ionization efficiencies and shifts of charge state (Figure 6b).
Figure 6: Comparison of normal LC and SFC for the analysis of a protein mixture. (a) UV chromatograms obtained with a PolyPentyl A column at 25 °C using a normal LC gradient (upper panel) and a CO2 dual gradient (lower panel). (b) Mass spectra observed for ribonuclease A from bovine pancreas (RNaseA) and lysozyme from chicken egg white (Lys) using a normal LC gradient (upper panel) and a CO2 dual gradient (lower panel). Adapted from reference (51) with permission.
Overall, the major advantage of SFC–MS compared with LC–MS is its suitability for the simultaneous analysis of a wide range of compounds showing very different chemical properties. SFC–MS offers excellent flexibility, due to the large diversity of commercially available stationary phases and the multiple options possible for the composition of the mobile phase. Together with the ongoing instrumentation development and improvements, SFC–MS is expected to play a major role and become increasingly used in multiple analytical applications.
Size-Exclusion Chromatography (SEC)-MS for the Analysis of Synthetic Polymers and Non-Covalent Protein Complexes
Size-exclusion chromatography (SEC) separates molecules according to their hydrodynamic volume (or size) based on the difference in exclusion from the pores of the stationary phase. Under ideal SEC conditions (where there is no interaction of the solutes with the stationary phase), the retention time can be translated to molecular weight using appropriate calibration standards. SEC–MS is considered an attractive tool for the analysis of mixtures of molecules showing a wide molecular weight distribution, such as synthetic polymers and protein complexes. Although chemically different, proteins and synthetic polymers share the characteristics of being heterogeneous, distributing in an ensemble of polymer-forms, differing in properties such as chemical composition, molecular weight, and higher order structure organization.
Synthetic polymers are chemical products used in a large diversity of everyday life applications, such as plastics and coatings. Polymers are produced through polymerization of chemical units (referred to as monomers), resulting in a heterogeneous distribution of molecules. The development of high performance polymers (used in applications such as drug delivery systems) relies on tailoring the distribution of chemical features to achieve the desired product properties. Such chemical features include molecular weight distribution, end-group functionality, topology (branching vs. cyclic), and polymer sequence distribution.
SEC-UV is typically used to determine the molecular-weight distribution of soluble polymeric products, but it does not provide any information on the molecular composition of the sample. On the other hand, MS can provide information on multiple chemical distributions (52). Therefore, the combination of SEC with MS is highly relevant in the field of polymer analysis. However, the common mobile phase systems used in SEC-UV (apolar solvents like THF) are not compatible with ESI-MS. An interesting approach is to couple SEC–MS using a post-column make-up flow (53). In this set-up, the LC eluent is mixed post-column with an organic solvent (such as methanol containing an ionization agent such as NaI) (54). Furthermore, supercharging agents can be used as additives to improve the ionization and detect higher molecular weight (MW) polymers showing a wide variety of chemistries (such as polystyrene, acrylates, and polyesters), also enabling the ionization of hydrophobic polymers (55).
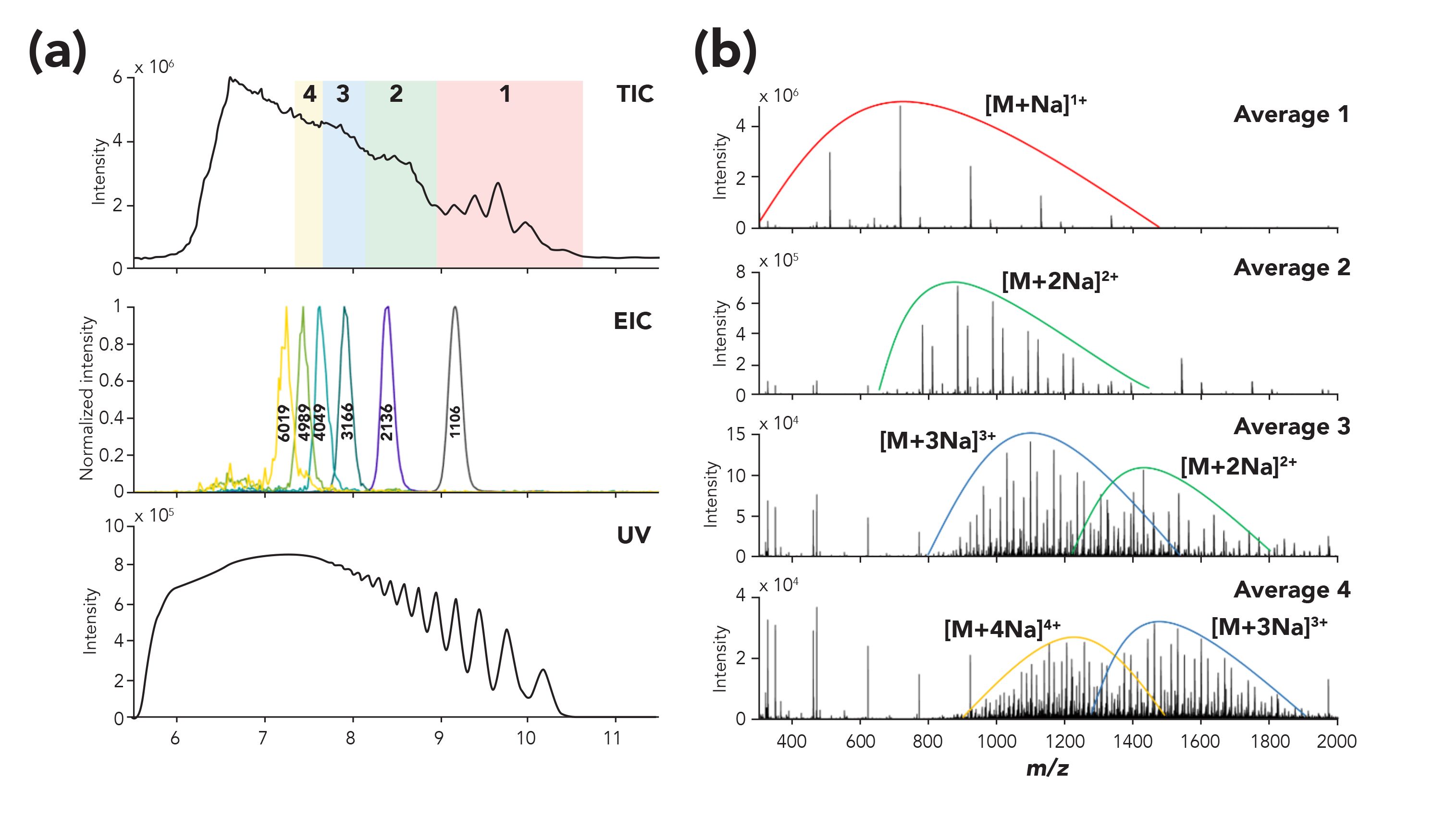
The combination of the resolving power of SEC, partly discriminating the sample polydispersity, with ESI-MS with supercharging agents significantly extends the MW range that can be measured. As a result, SEC–MS with supercharging agents allow for the analysis complex synthetic polymers up to 10 kDa range. Figure 7 shows an example of SEC–MS for the analysis of a branched polyester resin sample (unpublished data from Groeneveld and associates [56]). Species up to [M + 6Na]6+ were observed, allowing for the detection of species up to 6 kDa distributed in a highly complex MS spectrum.
Figure 7: Analysis of an industrial branched polyester resin sample using SEC–MS. Left panel: total ion current chromatogram (TIC), extracted ion chromatogram (EIC) for polymers of different masses and UV trace. Right panel: average mass spectra collected at different time intervals, as indicated in the colored boxes of the TIC. Annotation highlights the increase of charge states for higher mass species detected at earlier retention times. Supercharging conditions are described in reference (55,56).
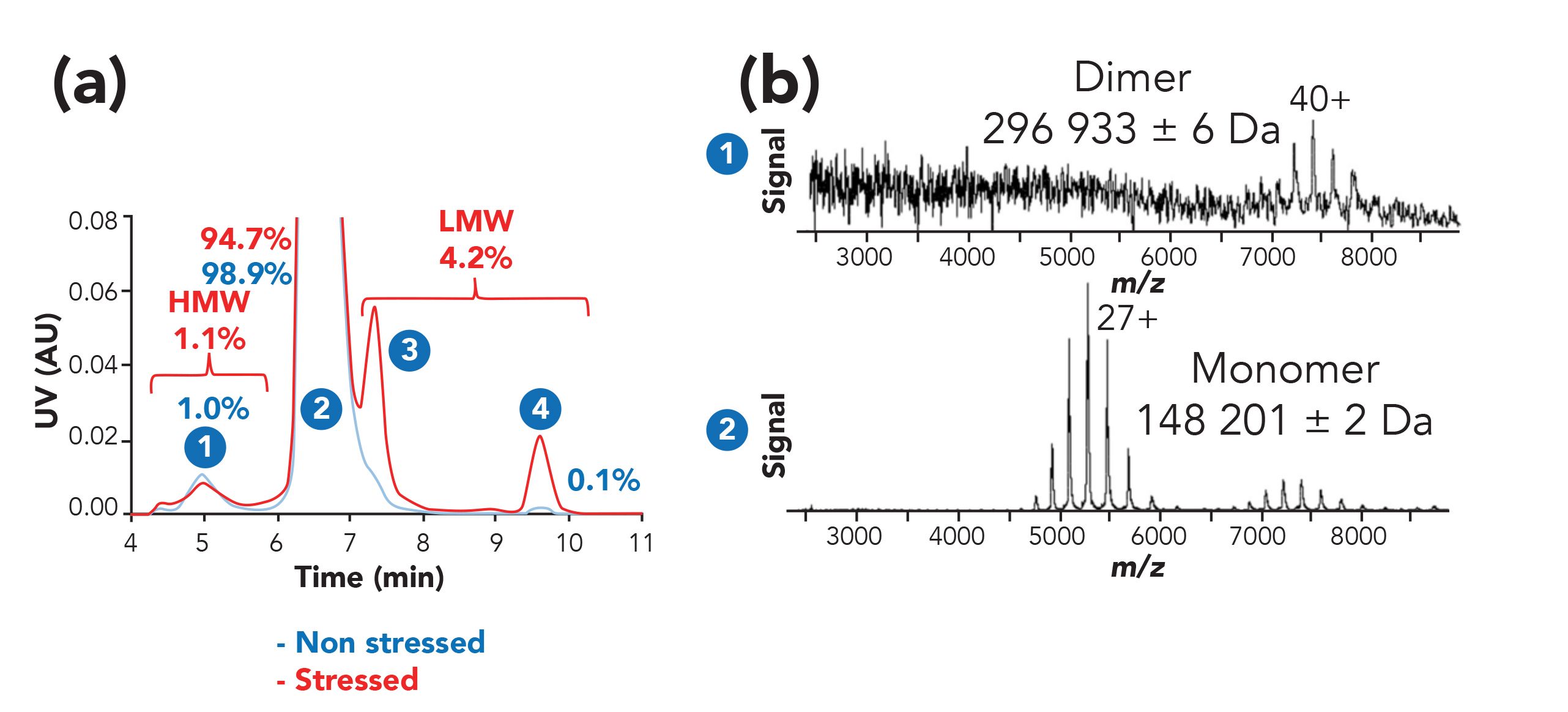
Beside polymer characterization, SEC–MS is often used for the characterization of biotechnological products, notably native SEC–MS, which is gaining more attention. Size and conformation based separation are of interest as often the active form of a protein is determined by its higher order structure distribution (dimerization). The information on the protein structural organization is lost when using denaturing methods such as reversed-phase LC and HILIC for the analysis of biopharmaceuticals. Native (or non-denaturing) separation approaches are indeed only accessible using mild conditions, such as water-based solutions at neutral pH and separation at room temperature. Coupling native SEC to MS allows for the analysis of protein complexes and aggregates. Under native separation conditions, water-based mobile phases with relatively high concentrations (100 mM) of volatile salts are used. These conditions result in a mitigation of ionic interactions with the stationary phase and maintain non-covalent interactions during measurements, enabling to obtain protein structural information (57). An example is illustrated in Figure 8 with the analysis of monoclonal antibodies (mAbs), where dimer, monomers and mAbs fragments of are separated using SEC–MS (58). Similar workflows can also be used to desalt and buffer-exchange protein products, allowing for the analysis of very large molecular complexes (59).
Figure 8: Online SEC-native MS analysis of a temperature-stressed NIST mAb sample. (a) Overlaid UV chromatogram of stressed (red trace) and unstressed (blue trace) NIST mAb. (b) Native mass spectra of peaks (1) and (2) observed for the SEC analysis of the temperature-stressed NIST mAb sample. Figure adapted from reference 58 with permission.
The (Not Necessarily) Salty: Ion-Exchange Chromatography (IEC) and Hydrophobic Interaction Chromatography (HIC)–MS
The growing interest in characterizing protein-based biotechnological products in their intact forms under nondenaturing conditions has fostered further developments in alternative chromatographic approaches such as ion-exchange chromatography (IEC), hydrophobic interaction chromatography (HIC) and capillary electrophoresis, focusing on the development of MS-compatible liquid-phase conditions.
IEC is commonly used to characterize charge variants of mAbs, such as those from amino acid variants, or PTMs such as deamidation. Typically, IEC methods are based on the use of cation-exchange stationary phases and mobile-phase gradients where either the concentration of salts (NaCl) is increased during analysis, or the pH is changed during analysis. Reference methods however make use of nonvolatile buffers or salt components; they are therefore not suitable for direct MS coupling and can only be used combined with desalting procedures.
Recent results, such as reported by Füssl and associates (60,61), have demonstrated the feasibility of performing pH gradient-based IEC separations using low concentrations of volatile salts such as ammonium acetate. These results underline the importance of a separation step to obtain a detailed characterization of molecules having similar masses (62,63). Figure 9 illustrates a striking example of the analytical power of such method, with the separation of the deamidated forms of the mAb trastuzumab, differing in only 1 Da on a molecule with a MW of ca. 150 kDa (64)! This detailed characterization is not possible with direct MS (65) or using denaturing reversed-phase LC–MS methods, as they lack the sufficient selectivity to differentiate the charge variants.
Figure 9: Analysis of trastuzumab using IEC-MS. (a) Simulated spectrum for the deamidated (†) and relatively unmodified (*) isoforms of trastuzumab G0F/G0F 27+ charge state. The centroid peak apices of the two isoforms are distinguished by a calculated mass difference of 6.75 ppm. (b) Experimentally measured m/z values for deamidated and unmodified G0F/G0F 27+ ions, shown as profile and centroid spectra. (c) Base peak chromatogram (BPC, upper panel) with labeled peaks corresponding to deamidated and relatively unmodified isoforms, extracted ion chromatogram (XIC, lower panels) traces for deamidated and unmodified isoforms rounded to nearest hundredth m/z value and plotted using a 3.3-ppm extraction width. (d) Major isoforms (corresponding to the top three glycan combinations ± deamidation) are detected using Sliding Window deconvolution and plotted as individual extracted deconvoluted mass traces (3.3 ppm mass/merge tolerances). Reproduced from reference (64) with permission.
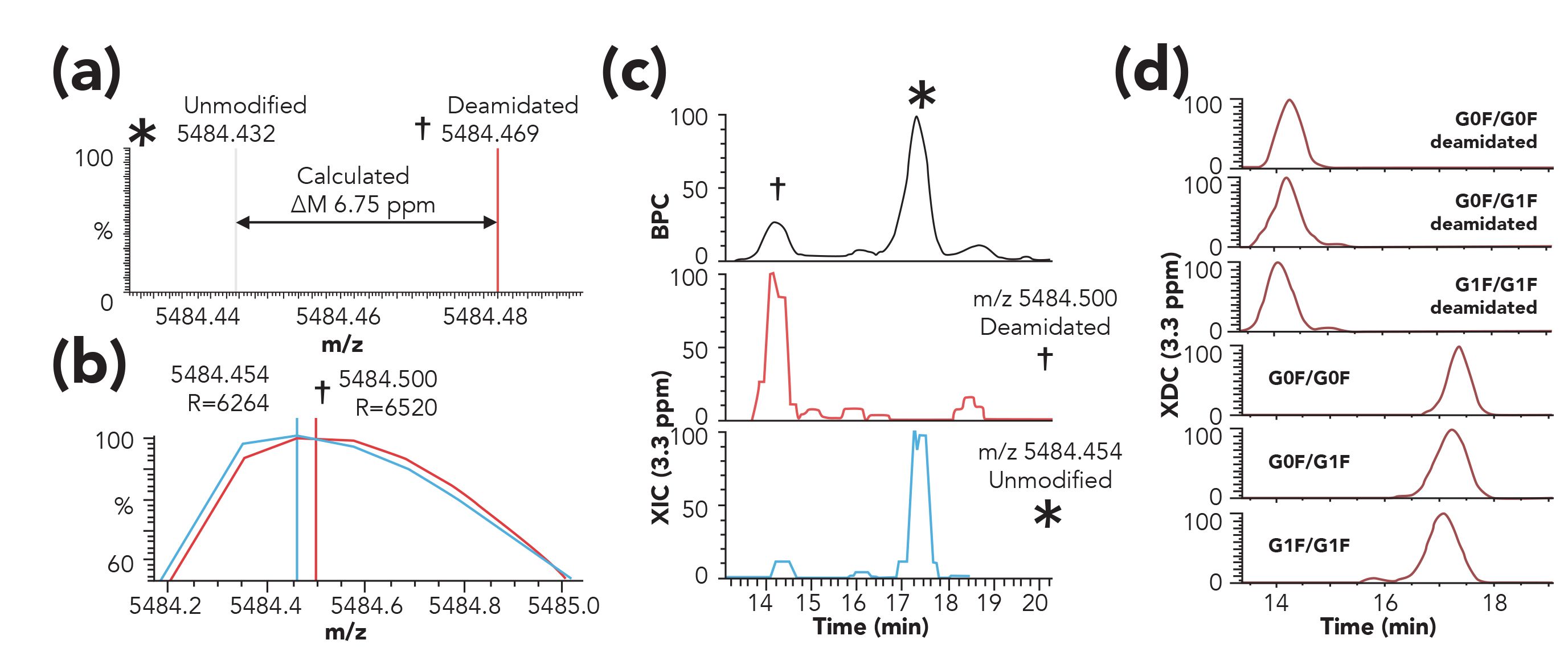
Trends in this field focus on the development of optimized methods to further enhance the overall sensitivity and widen the applicability of IEC-MS to other protein-based therapeutics, as well as other biotechnological products.
HIC separates proteins based on the hydrophobicity of the residues exposed in the protein tertiary structure under nondenaturing conditions, allowing for the preservation of noncovalent interactions. Similar to IEC, nonvolatile salts (ammonium sulphate, for example) are commonly used in HIC, but Bifan and colleagues have demonstrated that ammonium acetate in combination with small percentages of organic solvents is suited for HIC–MS analysis (66). However, with this volatile salt, most of the proteins are not retained using conventional HIC columns. Interestingly, a relatively wide spectrum of proteins can be retained using columns functionalized with selectors with higher hydrophobic character, for instance with selectors with a longer chain length. In their study, the authors clearly demonstrated the feasibility of HIC–MS, showing relevant examples in the context of top-down proteomics analysis (66) and analysis of mAb samples (67). These convincing proof-of-principle results require follow-up studies where further steps are taken to reduce the amount of salt used (here over 1M) and improve the separation efficiency.
Conclusions
Reversed-phase LC–MS has long remained the gold standard chromatographic technique for the analysis of a large diversity of compounds, from metabolites to biopharmaceuticals via lipids, glycans, and peptides. Over the last decade, remarkable technological developments have been carried out in the fields of HILIC, SFC, SEC, IEC, and HIC, fostering their use in many different applications. Those developments include the commercialization of novel stationary phase chemistries and new instruments, as well as experimental conditions enabling the direct coupling to MS. Overall, these innovations have raised the interest of the analytical science community, seeking alternative chromatographic options that would help solving the challenges encountered with reversed-phase LC–MS. Moreover, alternative selectivities may be a valuable tool to address analytical questions when not having access to the latest MS technology platforms.
Most of the alternative chromatographic techniques are now reaching level of maturity that allow them to be used outside academic research. HILIC, SFC, SEC, IEC, and HIC, together with important liquid-based separation approaches that were not covered here, such as capillary electrophoresis, multi-dimensional liquid chromatography, normal phase chromatography and chiral chromatography, are not expected to replace reversed-phase LC–MS, but will become increasingly important in analytical sciences, enabling the scientific community to tackle analytical challenges that cannot be entirely solved with reversed-phase LC alone. We, therefore, find it essential that the new generation of young scientists become familiar with those techniques as early as possible, together with reversed-phase LC–MS.
References
- C.M. Whitehouse, R.N. Dreyer, M. Yamashita, and J.B. Fenn, Anal. Chem. 57(3), 675–679 (1985). https://doi.org/10.1021/ac00280a023.
- J.B. Fenn, M. Mann, C.K. Meng, S.F. Wong, and C.M. Whitehouse, Science 246(4926), 64–71 (1989). https://doi.org/10.1126/science.2675315.
- L. Lin, Q. Yu, X. Yan, W. Hang, J. Zheng, J. Xing, and B. Huang, Analyst 135(11), 2970–2978 (2010). https://doi.org/10.1039/c0an00265h.
- P.J. Taylor, Clin. Biochem. 38(4), 328–334 (2005). https://doi.org/10.1016/j.clinbiochem.2004.11.007.
- Y. Shen, N. Tolić, C. Masselon, L. Pasa-Tolić, D.G. Camp, K.K. Hixson, R. Zhao, G.A. Anderson, and R.D. Smith, Anal. Chem. 76(1), 144–154 (2004). https://doi.org/10.1021/ac030096q.
- P. Xiang, Y. Zhu, Y. Yang, Z. Zhao, S.M. Williams, R.J. Moore, R.T. Kelly, R.D. Smith, and S. Liu, Anal. Chem. (2020). acs.analchem.9b05639. https://doi.org/10.1021/acs.analchem.9b05639.
- V. Marx, Nat. Methods 16(9), 809–812 (2019). https://doi.org/10.1038/s41592-019-0540-6.
- Y. Zhu, P.D. Piehowski, R. Zhao, J. Chen, Y. Shen, R.J. Moore, A.K. Shukla, V.A. Petyuk, M. Campbell-Thompson, C.E. Mathews, R.D. Smith, W.J. Qian, and R.T. Kelly, Nat. Commun. 9(1), 1–10 (2018). https://doi.org/10.1038/s41467-018-03367-w.
- L.M. Mallis, A.B. Sarkahian, J.M. Kulishoff, and W.L. Watts, J. Mass Spectrom. 37(9), 889–896 (2002). https://doi.org/10.1002/jms.360.
- C. Seger and L. Salzmann, Clin. Biochem. (2020). https://doi.org/10.1016/j.clinbiochem.2020.03.004.
- X. Wang, W. Li, and H.T. Rasmussen, J. Chromatogr. A 1083(1–2), 58–62 (2005). https://doi.org/10.1016/j.chroma.2005.05.082.
- H.P. Nguyen and K.A.J. Schug, Sep. Sci. 31(9), 1465–1480 (2008). https://doi.org/10.1002/jssc.200700630.
- S.R. Needham, P.R. Brown, K. Duff, and D.J.Bell, J. Chromatogr. A 869(1–2), 159–170 (2000). https://doi.org/10.1016/S0021-9673(99)00986-3.
- I. Kohler, R.J.E. Derks, and M. Giera, LCGC Europe 29(2), 60–75. (2016)
- R. Haselberg, B.W.J. Pirok, A. Gargano, and I. Kohler, LCGC Europe 32(9), 465–480 (2019).
- D. McCalley, LCGC North Am. 38(3), 173–181 (2018).
- P. Hemström and K. Irgum, J. Sep. Sci. 29(12), 1784–1821 (2006). https://doi.org/10.1002/jssc.200600199.
- K.J. Fountain, J. Xu, D.M. Dieh, and D. Morrison, J. Sep. Sci. 33(6–7), 740–751 (2010). https://doi.org/10.1002/jssc.200900660.
- A. Periat, B. Debrus, S. Rudaz, and D. Guillarme, J. Chromatogr. A 1282, 72–83 (2013). https://doi.org/10.1016/j.chroma.2013.01.037.
- D.Q. Tang, L. Zou, X.X. Yin, and C.N. Ong, Mass Spectrom. Rev. 35(5), 574–600 (2016). https://doi.org/10.1002/mas.21445.
- J.L. Spalding, F.J. Naser, N.G. Mahieu, S.L. Johnson, and G.J. Patti, J. Proteome Res. 17(10), 3537–3546 (2018). https://doi.org/10.1021/acs.jproteome.8b00487.
- M. Schwaiger, H. Schoeny, Y. El Abiead, G. Hermann, E. Rampler, and G. Koellensperger, Analyst 144(1), 220–229 (2019). https://doi.org/10.1039/c8an01219a.
- M. Lange and M. Fedorova, Anal. Bioanal. Chem. (2020). https://doi.org/10.1007/s00216-020-02576-x.
- A.R. Costa, M.E. Rodrigues, M. Henriques, R. Oliveira, and J. Azeredo, J. Crit. Rev. Biotechnol. 34(4), 281–299 (2014). https://doi.org/10.3109/07388551.2013.793649.
- P. Zhang, S. Woen, T. Wang, B. Liau, S. Zhao, C. Chen, Y. Yang, Z. Song, M.R. Wormald, C. Yu, and P.M.Rudd, Drug Discov. Today 21(5), 740–765 (2016). https://doi.org/10.1016/j.drudis.2016.01.006.
- A.F.G. Gargano, L.S. Roca, R.T. Fellers, M. Bocxe, E. Domínguez-Vega, and G.W. Somsen, Anal. Chem. 90(11), 6601–6609 (2018). https://doi.org/10.1021/acs.analchem.8b00382.
- V. D’Atri, S. Fekete, A. Beck, M. Lauber, and D. Guillarme, Anal. Chem. 89(3), 2086–2092 (2017). https://doi.org/10.1021/acs.analchem.6b04726.
- V. D’Atri, E. Dumont, I. Vandenheede, D. Guillarme, P. Sandra, and K. Sandra, LCGC Europe 30(8), 424–434 (2017).
- A. Periat, S. Fekete, A. Cusumano, J.-L.L. Veuthey, A. Beck, M. Lauber, and D. Guillarme, J. Chromatogr. A 1448, 81–92 (2016). https://doi.org/10.1016/j.chroma.2016.04.056.
- E. Domínguez-Vega, S. Tengattini, C. Peintner, J. van Angeren, C. Temporini, R. Haselberg, G. Massolini, and G.W. Somsen, Talanta 184, 375–381 (2018). https://doi.org/10.1016/j.talanta.2018.03.015.
- S. Tengattini, E. Domínguez-Vega, C. Temporini, T. Bavaro, F. Rinaldi, L. Piubelli, L. Pollegioni, G. Massolini, and G.W. Somsen, Anal. Chim. Acta 981, 94–105 (2017). https://doi.org/10.1016/j.aca.2017.05.020.
- A.F.G. Gargano, O. Schouten, G. van Schaick, L. Roca, J.H. van den Berg-Verleg, R. Haselberg, M. Akeroyd, N. Abello, and G.W. Somsen, Anal. Chim. Acta 1109, 69–77 (2020). https://doi.org/10.1016/j.aca.2020.02.042.
- Z. Zhang, Z. Wu, and M.J. Wirth, J. Chromatogr. A 1301, 156–161 (2013). https://doi.org/10.1016/j.chroma.2013.05.076.
- A.G. Huckabee, C. Yerneni, R.E. Jacobson, E.J. Alzate, T.H. Chen, and M.J. Wirth, J. Sep. Sci. 40(10), 2170–2177 (2017). https://doi.org/10.1002/jssc.201601376.
- M.J. Wirth, R.E. Jacobson, E. Jhovany, and A. Rodriguez, Anal. Sci. (2018).
- V. Pilarova, K. Plachka, M.A. Khalikova, F. Svec, and L. Novakova, Trac-Trends Anal. Chem. 112, 212–225 (2019). https://doi.org/10.1016/j.trac.2018.12.023.
- G.L. Losacco, J.L. Veuthey, and D. Guillarme, Trac-Trends Anal. Chem. 118, 731–738 (2019). https://doi.org/10.1016/j.trac.2019.07.005.
- C.G.A. da Silva and C.H. Collins, Quim. Nova 37(6), 1047-U210 (2014). https://doi.org/10.5935/0100-4042.20140158.
- E. Lesellier, and C. West, C. J. Chromatogr. A 1382, 2–46 (2015). https://doi.org/10.1016/j.chroma.2014.12.083.
- L. Novakova, A.G.G. Perrenoud, I. Francois, C. West, E. Lesellier, and D. Guillarme, Anal. Chim. Acta 824, 18–35 (2014). https://doi.org/10.1016/j.aca.2014.03.034.
- A.G.G. Perrenoud, D. Guillarme, J. Boccard, J.L. Veuthey, D. Barron, and S. Moco, J. Chromatogr. A 1450, 101–111 (2016). https://doi.org/10.1016/j.chroma.2016.04.053.
- D. Guillarme, V. Desfontaine, S. Heinisch, and J.L. Veuthey, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1083, 160–170 (2018). https://doi.org/10.1016/j.jchromb.2018.03.010.
- H. Takeda, Y. Izumi, M. Takahashi, T. Paxton, S. Tamura, T. Koike, Y. Yu, N. Kato, K. Nagase, M.Shiomi, and T. Bamba, J. Lipid Res. 59(7), 1283–1293 (2018). https://doi.org/10.1194/jlr.D083014.
- X. Xu, J.M. Roman, T.D. Veenstra, J. Van Anda, R. Ziegler, and H.G. Issaq, J. Anal. Chem. 78(5), 1553–1558 (2006). https://doi.org/10.1021/ac051425c.
- F. Petruzziello, A.G.G. Perrenoud, A. Thorimbert, M. Fogwill, and S. Rezzi, Anal. Chem. 89(14), 7615–7622 (2017). https://doi.org/10.1021/acs.analchem.7b01476.
- S. Bieber, G. Greco, S. Grosse, and T. Letzel, Anal. Chem. 89(15), 7907–7914 (2017). https://doi.org/10.1021/acs.analchem.7b00859.
- V. Desfontaine, G.L. Losacco, Y. Gagnebin, J. Pezzatti, W.P. Farrell, V. Gonzalez-Ruiz, SRudaz, J.L. Veuthey, and D. Guillarme, J. Chromatogr. A 1562, 96–107 (2018). https://doi.org/10.1016/j.chroma.2018.05.055.
- G.L. Losacco, O. Ismail, J. Pezzatti, V. Gonzalez-Ruiz, J. Boccard, S. Rudaz, J.L. Veuthey, D. Guillarme, J. Chromatogr. A 1620, 461021 (2020).
- K. Taguchi, E. Fukusaki,and T. Bamba, J. Chromatogr. A 1362, 270–277 (2014). https://doi.org/10.1016/j.chroma.2014.08.003.
- V. Desfontaine, F. Capetti, R. Nicoli, T. Kuuranne, J.L. Veuthey, and D. Guillarme, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1079, 51–61 (2018). https://doi.org/10.1016/j.jchromb.2018.01.037.
- Y. Wang and S.V. Olesik, Anal. Chem. 91(1), 935–942 (2019). https://doi.org/10.1021/acs.analchem.8b03970.
- C. Wesdemiotis, Angew. Chemie - Int. Ed. 56(6), 1452–1464 (2017). https://doi.org/10.1002/anie.201607003.
- K. Jovic, T. Nitsche, C. Lang, J.P. Blinco, K. De Bruycker, and C. Barner-Kowollik, Polym. Chem. 10(24), 3241–3256 (2019). https://doi.org/10.1039/c9py00370c.
- T. Gruendling, M. Guilhaus, and C. Barner-Kowollik, Anal. Chem. 80(18), 6915–6927 (2008).
- J. Steinkoenig, M.M. Cecchini, S. Reale, A.S. Goldmann, and C. Barner-Kowollik, Macromolecules 50(20), 8033–8041 (2017). https://doi.org/10.1021/acs.macromol.7b02018.
- G. Groeneveld, A.F.G. Gargano, T.S. Bos, R.L.C. Voeten, R.A.H. Peters, and P. Schoenmakers (in preparation)
- I.K. Ventouri, D.B.A. Malheiro, R.L.C. Voeten, S. Kok, M. Honing, G.W. Somsen, and R. Haselberg, Anal. Chem. (2020). https://doi.org/10.1021/acs.analchem.9b04961.
- A. Ehkirch, O. Hernandez-Alba, O. Colas, A. Beck, D. Guillarme, and S. Cianférani, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1086, 176–183 (2018). https://doi.org/10.1016/j.jchromb.2018.04.010.
- Z.L. VanAernum, F. Busch, B.J. Jones, M. Jia, Z. Chen, S.E. Boyken, A. Sahasrabuddhe, D. Baker, and V.H. Wysocki, Nat. Protoc. 15, 1132–1157 (2020). https://doi.org/10.1038/s41596-019-0281-0.
- F. Füssl, K. Cook, K. Scheffler, A. Farrell, S. Mittermayr, and J. Bones, Anal. Chem. 90(7), 4669–4676 (2018). https://doi.org/10.1021/acs.analchem.7b05241.
- Y. Leblanc, C. Ramon, N. Bihoreau, and G. Chevreux, J. Chromatogr. B 1048, 130–139 (2017). https://doi.org/10.1016/j.jchromb.2017.02.017.
- F. Füssl, A. Trappe, K. Cook, K. Scheffler, O. Fitzgerald, and J. Bones, MAbs 11(1), 116–128 (2019). https://doi.org/10.1080/19420862.2018.1531664.
- Y. Yan, A.P. Liu, S. Wang, T.J. Daly, and N. Li, Anal. Chem. 90(21), 13013–13020 (2018). https://doi.org/10.1021/acs.analchem.8b03773.
- A.O. Bailey, G. Han, W. Phung, P. Gazis, J. Sutton, J.L. Josephs, and W. Sandoval, MAbs 10(8), 1214–1225 (2018). https://doi.org/10.1080/19420862.2018.1521131.
- Y. Yang, G. Wang, T. Song, C.B. Lebrilla, and A.J.R. Heck, MAbs 9(4), 638–645 (2017). https://doi.org/10.1080/19420862.2017.1290033.
- B. Chen, Y. Peng, S.G. Valeja, L. Xiu, A.J. Alpert, and Y. Ge, Anal. Chem. 88(3), 1885–1891 (2016). https://doi.org/10.1021/acs.analchem.5b04285.
- B. Chen, Z. Lin, A.J. Alpert, C. Fu, Q. Zhang, W.A. Pritts, and Y. Ge, Anal. Chem. 90(12), 7135–7138 (2018). https://doi.org/10.1021/acs.analchem.8b01865.
Isabelle Kohler, Mingzhe Sun, Gino Groeneveld, and Andrea F.G. Gargano are with the Centre for Analytical Sciences Amsterdam, in Amsterdam, in The Netherlands. Kohler is with the Division of BioAnalytical Chemistry in the Amsterdam Institute of Molecular and Life Sciences, at Vrije Universiteit Amsterdam, in Amsterdam, in The Netherlands. Sun, Groeneveld, and Gargano are with the van ‘t Hoff Institute for Molecular Science, at the University of Amsterdam, in Amsterdam, in The Netherlands. Direct correspondence to: i.kohler@vu.nl or a.gargano@uva.nl

In the present study, a gradient reversed-phase high-performance liquid chromatography (RP-HPLC) method has been designed and validated to quantify ornidazole (OZ) in the marketed formulation (oral gel) with the application of QbD.
A column with chemically modified column hardware showed improvements in analytical performance for siRNA compared to a conventional stainless-steel column.