3 μm Particle-Based Chiral Stationary Phases for the Fast and Efficient Resolution of Enantiomers
LCGC Europe
The move from conventional particle sizes (5 μm or higher) to smaller diameter packing materials is one of the most attractive approaches to achieve higher separating efficiency. Recently developed 3 μm polysaccharide-derived chiral stationary phases demonstrate characteristics of favourable mass transfer kinetics, high column efficiency and good column permeability. This allows the fast analysis of enantiomers using conventional HPLC instruments.
The field of chromatographic analysis has evolved towards high throughput screening (HTS) in recent years. This trend has favoured the development of techniques and materials that allow shorter analysis times with high efficiency.
The separation of enantiomers now also requires a high throughput environment with the goal of developing analytical methods that completely resolve two peaks in the shortest time possible while preserving high efficiency. In practice, high speed chromatographic runs can be accomplished by adjusting certain chromatographic variables, such as flow-rate, temperature, column dimension and particle size. Reducing the particle size of the chromatographic packing materials is considered to be the most straightforward approach to achieving fast and highly effective separations.1–3 The enhanced efficiency is mainly attributed to the reduced intraparticle mass transfer resistance.4,5 In theory, the plate count is inversely proportional to the particle diameter.
Resolution of enantiomers by liquid chromatography (LC) is predominantly performed by using chiral stationary phases (CSPs). One of the most widely used CSP-types is the silica-based polysaccharide derived chiral supports. Until recently chromatographic supports based upon 5 μm silica particles have been the most popular matrix to pack the columns of conventional formats which match to the conventional HPLC instruments. This is true in a general sense and also for the specific chiral analysis domain. However, for high throughput method development and high productivity of routine analysis of enantiomers in pharmaceutical and agricultural laboratories or industries, 3 μm CSPs have been explored to determine if they can provide rapid resolution of enantiomers.
The 3 μm polysaccharide-derived CSPs developed are based on the analogous 5 μm chiral supports. The 3 μm CSPs were introduced at the beginning of 2008 commencing with Chiralpak AD-3 and Chiralcel OD-3 for applications with organic mobile phases; Chiralpak AD-3R and Chiralcel OD-3R for applications with aqueous eluents (RP-mode). The chiral selectors, tris(3,5-dimethylphenylcarbamate) of amylose for Chiralpak AD-type and tris(3,5-dimethylphenylcarbamate) of cellulose for OD-type, are physically coated on the silica matrix. In this article, the potential of these 3 μm CSPs for high throughput applications in terms of resolution of enantiomers is examined. Their beneficial characteristics in column and method efficiency will be discussed with regard to the corresponding 5 μm materials.
Experimental
The analytical columns based on 3 μm silica gels, Chiralpak AD-3, Chiralpak AD-3R, Chiracel OD-3 and Chiralcel OD-3R were in two formats: 4.6 × 150 mm and 4.6 × 50 mm. However, a unique column size (4.6 × 150 mm) was used for the 5 μm analogues: Chiralpak AD-H, Chiralpak AD-RH, Chiralcel OD-H and Chiralcel OD-RH. All the columns investigated were supplied by Daicel Chemical Industries Ltd (Tokyo, Japan).
For organic mobile phases, a basic additive, diethylamine (DEA) or ethylenediamine (EDA) was added into the mobile phase for resolution of basic compounds, while an acidic additive, trifluoroacetic acid (TFA), was systematically combined to the resolution of acidic compounds. In reversed-phase (RP) mode, the composition of the aqueous component was variable in terms of the sample nature: pure water for neutral molecules; formic acidic solution at pH 2.0 for acidic analytes; 20 mM NH4HCO3 at pH 9.0 (adjusted by adding DEA) for basic solutes.
The chromatographic tests were performed on two Agilent (Massy, France) LC systems. One was used for columns packed with 3 μm CSPs and for the acquisition of data to trace Van Deemter plot of 5 μm packed columns as well. This system consisted of the following modules: Agilent 1100 Series QuatPump with vacuum degasser, Agilent 1100 Series well-plate autosampler, Agilent 1200 Series thermostatted column compartment, Agilent 1100 Series diode-array detector B with a high pressure micro flow cell (1.7 μL) and 0.12 mm i.d. flow capillaries.
The second system was mainly used for parallel testing of the 5 μm packed columns. Its configuration was similar to the first one except for the following modules: Smartline Column Thermostat, Agilent 1100 Series diode-array (DA) detector B with its standard flow cell (16 μL), and the original 0.17 mm i.d. flow capillaries.
Results
CSPs on 3 μm silica: Under the concept of smaller particles resulting in more efficient columns, the silica particle technology has quickly progressed from the traditional 5 μm, via 3 μm and now towards sub-2 μm particles. The 3 μm particles present enhanced chromatographic characteristics at increased flow-rate when compared with 5 μm particles without experiencing excessive pressure problems. However, the sub-2 μm particles stress the pressure limitations of conventional HPLC instruments, making a direct link to the complexity and the robustness of equipment to conduct high speed separations. Indeed, recent advances in LC instrumentation have led to the ultra-high pressure LC (UHPLC) units, which can work at a pressure of up to 1000 bar.3
However, as a result of the associated cost of such ultra-high pressure resistant systems, as well as a series of technical constrains, the number of laboratories equipped with the so-called ultra-high performance liquid chromatography (UHPLC) systems is still quite limited. Under such circumstances, 3 μm CSPs seem to be a good compromise to improve efficiency and speed in the resolution of enantiomers without having to resort to a specific UHPLC system. Some research work does confirm that separation performance comparable to UHPLC in terms of speed and efficiency may be achieved by mastering the chromatographic variables using columns packed with 3 μm particles.5,6
In the framework of resolution of enantiomers, what improvement in performance can be expected with 3 μm CSPs compared with conventional 5 μm chiral packing materials? Figure 1 shows the two Van Deemter plots traced with data obtained respectively on the 3 μm and the 5 μm columns. As the theory predicts, the column packed with the 3 μm CSP gives a flatter curve than that based on the 5 μm packing material. Thus, one can run faster flow-rates (proportional to linear velocity μ) on 3 μm materials with much less loss in peak efficiency. This would, undoubtedly, represent a significant gain in analysis speed as the run time decreases in proportion to the increase in flow-rate. On the other side, the lower position of the 3 μm curve confirms its higher separation efficiency.
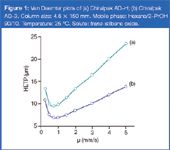
Figure 1
The addition of separating power as a result of the smaller particle size can be the most appreciable feature for resolution of difficult compounds. For instance, only a partial resolution of ibuprofen enantiomers could be achieved on Chiralpak AD-RH with the most effective screening mobile phase, whereas the two enantiomers were well separated at the same retention times on the corresponding 3 μm column under the same conditions [Figure 2(a) and (b)].
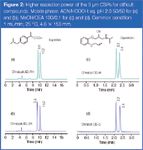
Figure 2
In the same line, the partial resolution of oxprenolol enantiomers on Chiralcel OD-H was transformed into full resolution on the corresponding 3 μm packed column using the same mobile phase [Figure 2(c) and (d)]. High column efficiency and favourable mass transfer kinetics are definitely the basic features of the 3 μm CSPs to gain their place in rapid and high performance resolution of enantiomers.
Method transfer from 5 μm packed to 3 μm packed columns: For two CSPs with the same chemical and physical compositions but just distinct from each other in particle sizes, capacity factors and enantioselectivity should be kept constant. The consistency in these two parameters is the key for direct method transfer.7
The good agreement in retentivity between the 3 μm and 5 μm packed columns can be exemplified in Table 1. The capacity factors k'1 of a series of compounds are virtually the same on each pair of columns, regardless of particle size.

Table 1
The consistency in enantioselectivity between the 3 μm and 5 μm CSPs is remarkable. A straight line with a tangent value approaching 1.0 and standard deviation equal to 0.998 was obtained through the scattered points of selectivity data (see Figure 3). This is a clear indication of the same enantioselective properties of the 3–5 μm CSP pairs under investigation.
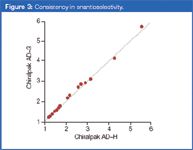
Figure 3
The good match of results between 3 μm and 5 μm CSPs makes method transfer straightforward. In Figure 4 two examples illustrate this aspect. The first is the resolution of racemic γ-(4 fluorophenyl)-γ-butyrolactone on the amylose-type selectors (same column dimension) with pure ethanol (EtOH) as the mobile phase. The same retention times could be reproduced on both columns [Figure 4(a) and (b)]. It is worth noting here the gain in resolution degree: Rs value was 1.3 times higher on the 3 μm column. The second example shows that retention times of propranolol enantiomers were almost the same on the cellulose-type selectors, but with enhancement of resolution by a factor of 1.4 on the 3 μm support [Figure 4(c) and (d)].

Figure 4
Effect of Temperature and Flow-Rate
As previously mentioned, increasing the flow-rate is one of the simpler approaches to achieving fast analysis. Most modern conventional HPLC instruments can support such an operation but within system pressure limits and in the flow-rate specifications of the instrument (usually 400 bar for 5 mL/min; 200 bar for flow-rates 5–10 mL/min). Furthermore, depending on the particle size, high flow-rate generates higher mass transfer resistance and thus may deteriorate the separation efficiency. Raising temperature to a certain extent may be a good strategy to overcome the efficiency loss without significantly affecting (or deteriorating in many instances) selectivity. In fact, chromatographic runs at elevated temperature have been advocated as a means of decreasing column back pressure, reducing the chromatographic run time and increasing column efficiency.4,8–14
The significance of temperature in the separation of enantiomers on 3 μm CSPs can be seen by the resolution of racemic indapamide. As displayed in Figure 5, the racemate was resolved into enantiomers on a short 3 μm column (25 °C, MeOH, 3 mL/min). As a result of the quite slow mass transfer kinetics of indapamide in such a chromatographic system, the plate counts of the two peaks were not very high: N1 = 1257; N2 = 609. Keeping the same temperature but increasing the flow-rate from 3 mL/min to 5 mL/min, approximately one third further loss in plate counts was detected. In contrast, by keeping the flow-rate at 3 mL/min and raising temperature up to 40 °C, the plate counts were enhanced and resolution was better than by simply increasing the flow-rate. Obviously, the temperature is an active variable for regulating the resolution of enantiomers and should be considered in combination with flow-rate in the search of the most efficient analytical method.

Figure 5
The separation of mephobarbital enantiomers on the 3 μm amylose column is a good example to demonstrate the combined effects of flow-rate and temperature in fast and high efficiency resolution. A very large resolution of racemic mephobarbital was observed within 4 min at 1 mL/min and 25 °C on a short column (4.6 × 50 mm, see Figure 6). More than 2 min between the two peaks are unnecessary for an analytical purpose. It could be narrowed by pushing the mobile phase through the column at 5 mL/min. Resolution degree is still 4.7 in these conditions and analysis time is reduced to less than 1 min. A five-fold increase in flow-rate led to a five times faster analysis. It is worth noting that the second eluting peak stays quite broad in the high flow-rate run, probably because of the relatively unfavourable mass transfer characteristics of the second enantiomer. In such a situation, a higher temperature would be able to improve the diffusivity between the mobile phase and the stationary phase [Figure 6(c)]. This temperature increase does not bring out major gain in analysis time, but contributes considerably to resolution degree. Such an approach should be considered when a chemical impurity issue is raised in the sample to be analysed and all impurities species have to be scratched out from the target molecule(s) in a single chromatogram.
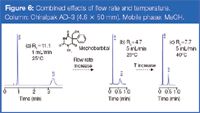
Figure 6
As concomitant effects of flow-rate and temperature, the run time is shortened from 4 min to 40 s by keeping 70% of the initial resolution degree. This practical example confirms that in some instances there is indeed very large room for method optimization, even for the separations undertaken on short columns.
Performance of the 3 μm CSPs for Fast Analysis
Polysaccharide-derived CSPs have been widely recognized to be versatile in the resolution of enantiomers. Their broad applicability is obviously attributable to their eminent selective performance for enantiomers of various natures. An enantioselectivity being large enough is undoubtedly the prerequisite to develop fast analysis method. The combination of 3 μm particle size with the polysaccharide derived selector would be a step forward in the search of rapid and highly efficient separation of enantiomers.

Figure 7
Owing to the generally favourable mass transfer characteristics of small particles, the chromatographic runs at given temperature and increased flow-rates are mostly featured by high efficiency and shorter analysis time. One example can be given by the resolution of 2-methyl tetralone enantiomers under RP conditions on a Chiralpak AD-3R column (4.6 × 50 mm). By visual examination, the two separations presented in Figure 7 seem to be identical in terms of peak shape and resolution, while run time is half in the second run. The change in flow-rate (from 0.5 mL/min to 1 mL/min) practically had no impact on the plate counts in this instance. Many resolutions of enantiomers can actually be done in the time range of few minutes or even shorter.
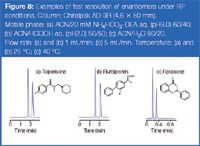
Figure 8
Some examples of fast resolution issuing from RP conditions are shown in Figure 8. It is worth noting that the separation of the two enantiomers of flavanone [Figure 8(c)] could be performed in sub-30 s. Such extremely high speed analysis is more frequently associated with mobile phases composed of organic solvents, as exemplified in Figure 9. The relatively low viscosity of most organic solvents enhances mass transfer in the mobile phase. In this scenario, one can make full use of the flow-rate capacity in the pressure limitation of the conventional HPLC instruments. As can be seen in Figure 9(d), as a result of the good column permeability, a flow-rate of 6 mL/min could be applied at 25 °C with the mixture of hexane/2-PrOH 90/10. In this instance, the total pressure was approximately 190 bar, but the column back pressure was only about 90 bar. The relatively low back pressure observed on the 3 μm packed column at high flow-rates allows the development of methods with very short run times. Further increase in flow-rate at 25 °C was unfeasible because the system pressure is limited to 200 bar with flow-rates superior to 5 mL/min, although mechanical stability of the particles has been proven at far higher pressure values.

Figure 9
Instrumental Considerations
As indicated in the first section, 3 μm packed columns can fit perfectly with the modern conventional HPLC instruments. For those old models built more than 10 years ago, however, it is important to reduce their relatively large dead volume in the flow path so that the advantages of 3 μm particles can be fully exploited. In fact, band broadening caused by too large extra column volume may be vulnerable to very sharp, fast eluting peaks. Old HPLC units may need an upgrade to adapt them to the applications mentioned. The whole aspects of HPLC upgrade is well covered by Huesgen in an application note which offers the guidelines for effective instrument upgrade operations.15
Conclusion
The newly developed 3 μm chiral stationary phases show certain advantages for analytical purposes. Their performance and HTS potential in routine chiral analysis, compared with the already existing 5 μm supports, are based on their higher column efficiency and more favourable mass transfer kinetics. These benefits on enantiomer resolution associated to the small particles are important for difficult separations and on gain of analysis time. Columns, especially short ones, packed with these 3 μm CSPs are able to afford rapid and high efficiency resolutions of enantiomers by making use of modern conventional HPLC instruments. The adequate adjustment in flow-rate and temperature is essential to bring the resolution of enantiomers into a new time dimension.
Acknowledgements
The authors wish to thank Mr Dung Nguyen for his remarkable contribution to the experimental work.
Tong Zhang received her PhD degree in Organic Chemistry from the University of Bordeaux-I (Bordeaux, France) in 1992. After three years postdoctoral work at Ciba-Geigy (Basel, Switzerland) under the supervision of Dr. Eric Francotte, she joined Chiral Technologies Europe (Strasbourg, France). She is now the R&D manager in the same company. Her main interests include development and applications of chiral stationary phases for resolution of stereoisomers by chromatography.
Pilar Franco received her PhD degree in Pharmacy from the University of Barcelona (Spain). After two years postdoctoral work in the University of Vienna (Austria) under the supervision of Professor Wolfgang Lindner, she joined Chiral Technologies Europe (Strasbourg, France). She is now Manager of Technical Operations in the same company. Her main interests include the applications of chiral selectors for the resolution of enantiomers at analytical and preparative scale.
References
1. H. Chen and C.S. Horváth, J. Chromatogr. A, 705, 3–10 (1995).
2. R.E. Majors, LCGC Eur., 19(6), 352–362 (2006).
3. S.A.C. Wren and P. Tchelitcheff, J. Chromatogr. A, 1119, 140–146 (2006).
4. F. Lestremau et al., J. Chromatogr. A, 1109, 191–196 (2006).
5. N. Sigari and J.W. Dolan, LC Troubleshooting Yearbook, 10–11 (2007).
6. K. Butchart, G. Foster and D. Temesi, Chromatography Today, 1, 3–7 (2007).
7. D. Guillarme, J.L. Veuthey and V.R. Meyer, LCGC Eur., 21(7), 322–327 (2008).
8. Y. Xiang, Y. Liu and M.L. Lee, J. Chromatogr. A, 1104, 198–202 (2006).
9. J.W. Dolan, LCGC Eur., 15(7), 394–397 (2002).
10. T. Greibrokk and T. Andersen, J. Chromatogr., 1000, 743–755 (2003).
11. D. Nguyen et al., J. Chromatogr. A, 1167, 76–84 (2007).
12. D.R. Stoll, J.D. Cohen and P.W. Carr, J. Chromatogr. A, 1122, 123–137 (2006).
13. B.A. Jones, J. Liq. Chromatogr. Relat. Technol., 27, 1331–1352 (2004).
14. D. Guillarm, S. Heinisch and J.L. Rocca, J. Chromatogr. A, 1052, 39–51 (2004).
15. A.G. Huesgen, Application Note, Agilent Technologies, October (2006).

HILIC Peptide Retention Times Predicted Using New Approach
October 29th 2024Manitoba Centre for Proteomics and Systems Biology scientists produced a new means of predicting peptide retention times for hydrophilic interaction liquid chromatography (HILIC) at acidic pH in formic-acid based eluents.