Two Novel Polysaccharide-Based Chiral Stationary Phases: CHIRALPAKˆ® AY-H and CHIRALCELˆ® OZ-H
Polysaccharide-based chiral stationary phases (CSP) are successfully employed to achieve the majority of analytical and preparative enantioselective separations. However, there are a number of separations where significant modification or enhancement of selectivity is necessary. Two new columns, CHIRALPAK AY-H and CHIRALCEL OZ-H, have been introduced to meet these needs.
Geoffrey B. Cox and Norbert M. Maier, Chiral Technologies, Inc.
Polysaccharide-based chiral stationary phases (CSP) are successfully employed to achieve the majority of analytical and preparative enantioselective separations. However, there are a number of separations where significant modification or enhancement of selectivity is necessary. Two new columns, CHIRALPAK AY-H and CHIRALCEL OZ-H, have been introduced to meet these needs.
Chiral stationary phases based on methylchlorophenyl carbamates of cellulose (CHIRALCEL OZ) and amylose (CHIRALPAK AY and AZ), coated on 20-micron silica, have been available for some years for preparative separations. These CSPs have proven to be valuable in Chiral Technologies, Inc's custom separation service operations, generating results superior (~ 10% of the separations) to our more established CSPs. To exploit the enhanced selectivity of these complementary CSPs, 5-micron versions have been developed for analytical and semi-preparative use.
Structure
The new 5-micron phases are based upon the same wide-pore silica as is used for the other Daicel media. CHIRALPAK AY-H is the tris-(5-chloro-2-methylphenylcarbamate) of amylose whereas CHIRALCEL OZ-H is tris-(3-chloro-4-methylphenylcarbamate) of cellulose. Scale-up from these 5-micron columns, if needed, to the 20-micron preparative phases can easily be accomplished.
Stability
Pressure stability for HPLC columns is increasingly critical as chromatographers develop faster applications. Pressure testing was carried out by running the columns at pressure drops up to 250 bar over a period of three weeks. No change in performance was observed during these tests.
Selectivity
The chromatograms illustrate separations where the selectivity of the new columns is seen to be different from that of other, more established phases. Figure 1 shows a much enhanced separation of the enantiomers of methyl 1-benzyl-5-oxo-3 pyrrolidinecarboxylate using CHIRALCEL OZ-H (red trace) over that using CHIRALCEL OD-H (blue trace). Figure 2 shows the separation of diastereoisomers of the herbicide metolachlor in comparison with a recently developed separation using CHIRALCEL OD-3. The separation on CHIRALPAK AY-H shows a different elution order of the diastereomers as well as a marked reduction in analysis time.
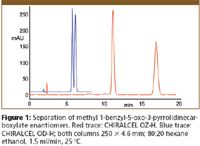
Figure 1
Conclusions
Novel CSPs based on 5-and 20-micron silica with methyl chlorophenylcarbamates have been developed. These new CSPs clearly demonstrate alternative selectivity to other polysaccharide-based chiral phases for both analytical and preparative applications.
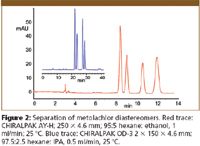
Figure 2
CHIRALPAK and CHIRALCEL and OD are registered trademarks; AY, AZ and OZ are trademarks of Daicel Chemical Industries, Ltd.

Chiral Technologies, Inc.
800 North Five Points Road, West Chester, PA 19380
tel. (800)6-CHIRAL
Website: www.chiraltech.com
