Rapid Field Detection of Chemical Warfare Agents, Simulants, By-Products, and Precursors Using Solid Phase Microextraction and Portable GC-TMS
The Application Notebook
Rapid sample preparation using the CUSTODIONâ„¢ solid phase microextraction (SPME) syringe was applied to chemical warfare agents (CWAs), CWA simulants, by-products, and precursors. The samples were analyzed quickly and reliably with a sample-to-sample cycle time of less than 3 min using the GUARDIONâ„¢-7 portable capillary gas chromatograph toroidal ion trap mass spectrometer (GC–TMS).
Rapid sample preparation using the CUSTODION™ solid phase microextraction (SPME) syringe was applied to chemical warfare agents (CWAs), CWA simulants, by-products, and precursors. The samples were analyzed quickly and reliably with a sample-to-sample cycle time of less than 3 min using the GUARDION™-7 portable capillary gas chromatograph toroidal ion trap mass spectrometer (GC–TMS).
Rapid and accurate detection of chemical warfare agents (CWAs), CWA precursors, and CWA by-products is imperative for human protection in warfare and security domains. Gas chromatography–mass spectrometry (GC–MS) is a selective and sensitive technique routinely used in laboratory settings. Now, GC–MS can be applied for near-real time detection of CWAs over a broad concentration range in situations requiring mobile analysis using a hand-portable GUARDION™-7 GC–TMS instrument in the field. Rapid sample preparation for air, headspace, liquids, and dissolved solid samples is accomplished by solid phase microextraction (SPME). In this application, low concentrations of CWAs, and low to neat (100%) concentrations of CWA precursors, simulants, and by-products were sampled and identified in less than 3 min.
Experimental Conditions
Six CWAs including VX, HD, HN3, GA, GB, and GD were prepared at 100 μg/ml in isopropyl alcohol. A CUSTODION SPME syringe with a 65 μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber was used for extraction by immersing the fiber directly into the sample for 5 s. The CWA simulants, by-products, and precursors including diisopropylmethylphosphonate (DIMP), methyl salicylate (MES), tributylphosphate (TBP), pinacolyl alcohol (PA), diethylmalonate (DEM), and dimethylmethylphosphonate (DMMP) were sampled in separate analytical runs at concentrations ranging from trace to neat (100%) for 5 s.
Following each sample preparation, the SPME syringe was inserted into the GUARDION-7 GC-TMS injection port where the target analytes were desorbed into a low thermal mass injector (275 °C) coupled with a metal-clad capillary GC column (MXT-5, 5 m × 0.1 mm, 0.4 μm df). After an initial 10 s hold at 50 °C, the GC temperature was increased at 2 °C/s to 280 °C. The capillary GC is coupled to a TMS detector having a mass range of 45–500 m/z. A user defined compound library identified the target analytes using an on-board deconvolution algorithm.
Results
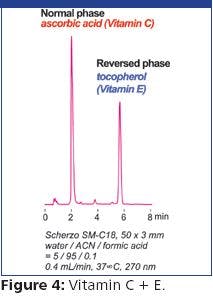
Figure 1 shows the GC–TMS chromatogram and mass spectrum for VX prepared in isopropyl alcohol after preparation at below regulated surety level. VX was positively identified by the GUARDION-7 in 130 s.
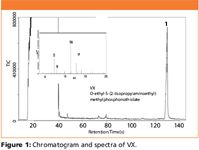
Figure 1
Conclusions
The CUSTODION™ SPME syringe and GUARDION™-7 GC–TMS are uniquely suited for field or laboratory screening of CWAs and other organic compounds to support rapid decision making. The ability to analyze a wide variety of sample types at concentrations from trace to neat allows the CUSTODION SPME syringe and GUARDION-7 GC–TMS to provide near real-time field analysis results. The short cycle time between injections allows the user to quickly analyze samples on-site with high sensitivity and specificity.
Acknowledgements
CUSTODION™ and GUARDION™ are registered trademarks of Torion Technologies Inc. The CUSTODION SPME syringes are manufactured and sold under license from (1) Brigham Young University under US Patent Application 11/379,716 and (2) SUPELCO under US Patent 5,691,206, and/or any divisions, continuations, or revisions thereof.

Torion Technologies Inc.
796 East Utah Valley Drive, Suite 200, American Fork, UT 84003
tel. (801)705-6600
Website: www.torion.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis